Abstract
Thirty to fifty percent of critically ill patients admitted to the intensive care unit suffer from generalized neuromuscular weakness due to critical illness polyneuropathy, critical illness myopathy, or a combination of them, thus prolonging mechanical ventilation and their intensive care unit stay. A distinction between these syndromes and other neuromuscular abnormalities beginning either before or after ICU admission is necessary. These intensive care unit-related diseases are associated with both elevated mortality rates and increased morbidity rates. Generally, over 50 % of patients will completely recover. Most of them recover after 4–12 weeks, but some patients have been reported to keep on suffering from muscle weakness for at least 4 months. Prevention has a key role in the management of critical illness neuromuscular disorders, as no specific therapy has been suggested. Either prevention or aggressive treatment of sepsis can prevent critical illness polyneuropathy and critical illness myopathy. The dose and duration of the administration of neuromuscular blocking drugs should be limited, and their concurrent administration with corticosteroids should be avoided. Intensive insulin therapy has also been proven to reduce their incidence. Finally, early mobilization via active exercise or electrical muscle stimulation plays a significant role in their prevention.
Similar content being viewed by others
Introduction
Over the last two decades, generalized neuromuscular weakness has constituted a severe, frequent, and persistent complication among critically ill patients admitted to the intensive care unit (ICU) due to infection, trauma, surgery, and burns [1–3]. Moreover, it is frequently responsible for prolonged mechanical ventilation [4] and prolonged ICU stay [5]. In 1977, MacFarlane and colleagues were the first to report critical illness-related severe weakness after neuromuscular blocking agents and corticosteroids administration in an asthmatic 24-year-old woman [6]. It was a little later, in 1984, when Bolton et al. [7] also observed severe polyneuropathy in five critically ill patients experiencing severe weakness and being dependent on mechanical ventilation. Newly acquired weakness in the ICU is mainly due to critical illness neuromuscular disorders including critical illness polyneuropathy (CIP) and critical illness myopathy (CIM) [3]. The former is characterized by both motor and sensory fiber degeneration, primarily concerning lower limb nerves [1], whereas the latter is characterized by fiber atrophy, fatty degeneration of muscle fibers, and fibrosis [8]. However, both of them lead to muscle weakness and paralysis in critically ill patients and they often coexist, making their differential diagnosis obscure [1]. Hereby, we will try to shed light on these dark clinical entities.
Pathophysiology
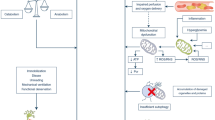
Patients with systemic inflammatory response syndrome and multiple organ dysfunction experiencing respiratory insufficiency are prone to CIP/CIM [9–11]. Inflammation, apoptosis, thrombosis, and oxidant injury constitute the basis of both multiple organ failure (MOF) and peripheral neuromuscular abnormalities [2], so neuromuscular weakness because of CIP/CIM is due to neural system participation in MOF [5]. According to Bolton et al. [12, 13], microcirculation damage in peripheral nerves and muscles triggered by sepsis has a critical role in the CIP/CIM pathogenesis.
Pro-inflammatory cytokines such as TNF-α and IL1 induce a raise in the levels of E-selectin expression [1] in endothelium of epineurial and endoneurial vessels promoting endothelial cell leukocyte activation [14]. Activated leukocytes locally generate cytokines, damaging tissues [1]. Furthermore, sepsis-related cytokine secretion enhances microvascular permeability [13]. Consequently, the blood–nerve barrier becomes more permeable, allowing circulating toxins to directly impair the axons [12]. Some cytokines are also a direct neurotoxic factor for peripheral nerves [15]. Hyperglycemia and reactive oxygen species are additional adverse factors against the peripheral nerve microcirculation [16]. This microcirculation disturbance harms peripheral nerve [17] and organ perfusion [18] and results in significant energy deficits inducing primary distal axonal degeneration [19], which is the major pathological feature of CIP [2].
Metabolic, inflammatory, and bioenergetic changes compose the pathophysiology of CIM [16]. CIM is characterized by proteolysis aided by pro-inflammatory cytokines and increased apoptosis [20], elevated urinary nitrogen levels [2], and skeletal muscle hypercatabolism in combination with the general hypermetabolism of critical ill patients [21, 22]. Low protein/DNA levels and high extracellular water concentrations are additional findings of a muscle biopsy in critically ill patients [2]. Muscle membranes are often proved to be unexcitable by electrodiagnostic studies in CIM [16]. According to Rich et al. [23], the reasons why this happens are lower muscle resting potential because of denervation (from −85 to −60 mV), the lack of postdenervation down-regulation of membrane chloride conduction, and the shift in the voltage dependence of sodium channel fast inactivation to more negative potentials (−11 mV) [24, 25]. Moreover, altered nitric oxide generation in skeletal muscle as well as mitochondrial dysfunction impairs muscle membrane excitability in CIM [1, 16].
Administered corticosteroids and neuromuscular blocking drugs (NMBDs) may also have an impact on the pathogenesis of CIP and CIM [2, 10, 26]. NMBDs seem to either have direct toxic effects on the nerve or stimulate functional denervation of the muscle due to the enhanced microvascular permeability [13]. Finally, they may favor toxic effects of corticosteroids or inflammatory mediators [13, 16]. Furthermore, pathophysiologic changes of diaphragm also participate in the pathophysiology of CIP and CIM. Hence, if the diaphragm is inactive and mechanical ventilation is used for 18–69 h, noticeable atrophy of diaphragm myofibers occurs [27].
Incidence
Overall, 30–50 % of critically ill patients suffer from CIP, CIM, or a combination of them [3]. Nevertheless, there are many factors strongly influencing the incidence of neuromuscular disorders, such as a patient’s case-mix, diagnostic criteria, timing of diagnosis [1, 28], ICU stay length, and severity of sepsis [3]. Hence, 70 % of patients experiencing sepsis or systemic inflammatory response syndrome (SIRS) will suffer from CIP [12] and 60 % of patients suffering from acute respiratory distress syndrome (ARDS) will acquire either CIP or CIM [29]. Moreover, MOF is associated with a 100 % CIP prevalence [9], as well as critically ill patients in coma, all of whom will manifest CIP or CIM [8]. Mechanical ventilation duration of 4–7 days leads to CIP/CIM in 25–33 % of patients if they are clinically evaluated [10, 30, 31] and up to 58 % if they are electrophysiologically evaluated [32–34]. Finally, ICU stay of at least 7 days is related to a 49–77 % CIP/CIM prevalence [35–37].
Diagnosis
Clinical evaluation of CIP/CIM
Female sex, age above than 50 years old, respiratory failure, SIRS, and ICU stay over 5 days are the typical characteristics of patients suffering from CIP/CIM [2]. CIP describes an acute axonal polyneuropathy in critically ill patients [1], whereas CIM refers to an acute, extensive, non-necrotizing myopathy in critically ill patients [1, 3]. Motor and sensory fiber degeneration primarily concerning lower limb nerves is the typical characteristic of CIP [1], whereas fiber atrophy, fatty degeneration of muscle fibers, and fibrosis are the main characteristics of CIM [8]. However, both lead to muscle weakness [1]. The main clinical features of CIP and CIM are flaccid and usually symmetrical weakness ranging from moderate paresis with reduced deep-tendon reflexes to severe quadriplegia with no deep-tendon reflexes, muscle wasting, and hypotonia with or without involvement of respiratory muscles and muscles innervated by cranial nerves [2, 3, 16]. Patients are often not capable of moving extremities in response to pain, while their strong facial grimaces show they are awake [3]. Contrary to most myopathies, weakness equally affects distal muscles as it does proximal ones [38]. Legs are influenced more frequently than arms [7, 39]. Weakness in ICU patients may escape our attention until flaccidity and wasting of extremities or mechanical ventilation dependency [3] caused by diaphragmatic or intercostal muscles weakness [40] are observed. Sensory impairment, including a distal loss of sensitivity to pain, temperature and vibration, can also occur in patients with CIP [16]. Generally, SIRS and multiorgan dysfunction followed by generalized or distal weakness, distal sensory deficits, spared cranial nerves, and findings of axonopathy on EMG are basic clinical signs of CIP, whereas generalized or proximal weakness, elevated creatine kinase, and myopathic findings on EMG after the administration of high doses of NMBDs and corticosteroids are common clinical features of CIM [3] (see Tables 1, 2).
Diagnosing CIP/CIM
Serum creatine kinase levels (CK), electromyography (EMG), nerve conduction studies, and muscle biopsy can help in the diagnosis of CIP/CIM [16]. Although CK level is often 10–100-fold increased in myopathy, making peak on the third or fourth day of the illness [3], it is usually normal in CIP and prolonged neuromuscular blockade [40], provided that muscle necrosis is absent or scattered [1]. After 10 days, the CK level is nearly normal even in CIM [3]. At least 15 % of patients present normal CK levels during their hospital stay [40]. Moreover, muscular traumas and needle EMG examination may cause CK levels to increase [3], as well as myoglobinuria and renal failure, which are associated with noticeable CK elevation [40]. Therefore, CK might not be a very useful parameter [16].
With regard to the electroneurographic pattern of CIP, the amplitude of the nerve action potentials (compound motor, sensory or both) is increased, whereas normal conduction velocity is maintained [1], only 3 days after onset of sepsis or ICU admission [30, 41]. On the contrary, reduced amplitude due to the reduced number of fibers and normal conduction velocity due to the normal myelin sheath of the rest of the fibers are typical of axonal neuropathy, whereas normal amplitude due to the normal number of fibers and reduced velocity due to demyelination characterize demyelinating neuropathy such as Guillain-Barré syndrome [1] (see Tables 1, 2).
These electroneurographic changes are prior to clinical signs as well as to denervation signs, such as fibrillation potentials and positive sharp waves, which may take place as late as during the second or third week of admission for critical illness [9, 42]. Nevertheless, these denervation signs may also be present in myopathies [43]. CIP is also characterized by bilateral reduced or absent diaphragm compound motor action potential in about 50–80 % [44, 45] and almost normal phrenic motor latency [12, 46, 47]. On the other hand, normal compound motor action potential, normal F wave latency, and normal repetitive stimulation response are typical of CIM [48].
According to Latronico and Rasulo [49] and several other investigators [10], the Medical Research Council scale or handgrip dynamometry are useful to reliably indicate the limb muscle strength of the ICU patient. Regarding the Medical Research Council score, three muscular groups of both upper and lower limbs are evaluated from 0 to 5 with regard to muscle force forming a maximum sum score of 60 [10, 16]. A Medical Research Council sum score below 48 or mean Medical Research Council score below 4 (antigravity strength), and a force value less than 11 kg-force for men and less than 7 kg-force for women at dominant-hand dynamometry proves the presence of ICU-acquired weakness in ICU patients without previous story of neuromuscular abnormalities [49]. Nevertheless, this score does not shed light on the reason why weakness exists and its reliability is limited as the patients’ wakeful status in order to cooperate is presupposed [16]. As far as respiratory muscle weakness is concerned, it can be diagnosed by evaluating the maximal inspiratory and expiratory pressures and vital capacity [49].
CIP or CIM
Differentiation between CIP and CIM is often not possible, as they often coexist, and their clinical, electrophysiological, and histological features cover each other [1–3, 8, 16, 42]. Muscle biopsy, being the gold standard for the involvement of muscles [16], provides strong evidence for the diagnosis [2]. Severity of myopathic changes varies in CIM characterized by thick myosin filament loss, while CIP is associated with neurogenic atrophy (see Tables 1, 2). However, muscle biopsy use is limited because of its invasiveness and it is usually used provided other causes of myopathy like polymyositis are possible [3]. According to Bolton’s study [13], muscle biopsy should be performed when clinical features suggest a myopathy. Stibler et al. [50] proposed that a percutaneous muscle biopsy obtained by a conchotome is a minimally invasive method that is useful for the rapid diagnosis of CIM in patients with decreased myosin-to-actin ratio.
Furthermore, direct muscle stimulation is an important diagnostic tool [2]. Conventional stimulation for example through the motor nerve will cause a reduced or absent action potential in patients with neuropathy. Direct muscle stimulation is related to a normal action potential in these patients. On the contrary, as far as myopathy is concerned, both conventional stimulation and direct muscle stimulation are associated with reduced or absent action potential [1]. According to Lefaucheur et al. [51], reduced direct muscle stimulation amplitude and motor nerve stimulation to direct muscle stimulation amplitude ratio over 0.5 or prolonged direct muscle stimulation latency are typical of myopathy, whereas neuropathy is characterized by a motor nerve stimulation to direct muscle stimulation amplitude ratio of less than 0.5. Additionally, polyneuropathy is not possible when normal sensory nerve action potentials (SNAP) amplitude is recognized whereas SNAP are preserved in myopathy [52].
Differential diagnosis
There are several disorders mimicking neuromuscular dysfunction related with critical illness which require a more specific therapy than CIP/CIM. These disorders can either pre-exist worsening during ICU stay or occur during ICU stay [2, 40]. Guillain-Barré syndrome, drug- and metabolism-induced neuropathies, myasthenia gravis [2], myopathy associated with hypokalemia, hypophosphatemia, or hypermagnesemia [40], uremia-related myopathy, and diabetes [2] constitute some of these disorders included in the differential diagnosis of CIP/CIM. Neuromuscular weakness can also be caused by acute myopathies because of rhabdomyolysis or acute polymyositis [3]. Brain stem infarct, hemorrhage, and central pontine myelinolysis leading to “locked-in” syndrome are additional causes of weakness included in the differential diagnosis of CIP/CIM. Acute transverse myelitis, epidural abscess, spinal cord infarct, traumatic spinal cord injury, and other spinal cord lesions causing generalized weakness should also be considered in the differential diagnosis [3].
Risk factors
There is an abundance of risk factors related to CIP and CIM (see Table 3). Severity of illness and ICU-stay length are two of these [10, 30, 34]. Sepsis, SIRS, multi-organ dysfunction syndrome, multi-organ failure, female sex, severe asthma, ionic abnormalities, malnutrition, immobility [1, 53], duration of organ dysfunction [10], central neurologic failure, renal failure and renal replacement therapy, hyperosmolality, parenteral nutrition [26], low serum albumin [12], and vasopressor and catecholamine support [36] have all been recognized to make critically ill patients more prone to critical illness neuromuscular abnormalities.
Whether corticosteroids impair the critically ill patients’ neuromuscular status is debatable [16]. The type and dose of corticosteroids themselves do not have any impact on the development of myopathy. On the other hand, neuromuscular blocking agents are an important risk factor for neuromuscular abnormalities development [2]. The majority of the neuromuscular blocking agents are metabolized by the liver and cleared by the kidneys. Hence, a long duration of neuromuscular blockade, lasting days or even weeks after discontinuation of drug administration, is possible to take place when large amounts of neuromuscular blocking drugs are administered to critically ill patients suffering from renal failure [6, 34]. Consequently, renal failure is a significant risk factor for prolonged neuromuscular blockade [2, 54, 55]. Hypermagnesemia, metabolic acidosis, and the concomitant use of various antibiotics including aminoglycosides and clindamycin also promote prolonged neuromuscular blockade but they are weaker risk factors than renal failure [2].
Hyperglycemia is also an important determinant of critical illness neuropathy and myopathy [2, 10, 56]. Finally, abnormal serum lipid profiles observed in critically ill patients also play a role in critical illness neuromyopathy, impairing normal function of some organs, including neural system [2, 57].
Prevention/management
Prevention has a key role in the management of critical illness neuromuscular disorders, since no specific therapy has been suggested yet (see Table 4) [40]. Either prevention or aggressive treatment of sepsis can prevent CIP/CIM [2]. According to Mohr et al. [58], early intravenous immunoglobulin (IVIg) administration in septic ICU patients may prevent CIP development. However, the IVIg efficacy is doubted by a previous study in three patients [59] demonstrating failure of IVIg to change the clinical course of critical illness polyneuropathy. Rehabilitation programs [60], managing mechanical ventilation dependency [61] and preventing neuropathies caused by pressure by careful patient positioning, also participate in the successful management of CIP/CIM [16]. Nutrition schemes and nutritional interventions [62], total parenteral nutrition [63], antioxidant therapy [64], and the use of testosterone derivates [65] and growth hormone [66] have also been applied to prevent CIP/CIM showing though no benefit with regard to muscle function [16].
Deep sedation caused by benzodiazepines (midazolam), propofol, and analgesics (fentanyl, morphine) is necessary for most critically ill patients in order to be mechanically ventilated [40]. Prolonged neuromuscular blockade should be prevented, so it is helpful to limit the dose and duration of NMBD administration, particularly in high-risk patients suffering from renal or hepatic failure [2]. Therapy with corticosteroids and NMBDs for over than 24–48 h grows the risk of acute myopathy [67], so the duration of their administration should be as short as possible [2]. Frequent interruptions in the drug administration of NMBDs in order to be cleared or pharmacologic reversal of neuromuscular blockade with a cholinesterase inhibitor can be useful to reveal a patient’s movement, which is necessary to assure the absence of prolonged neuromuscular blockade in the ICU [2]. Furthermore, the concurrent administration of corticosteroids and NMBDs should be avoided as much as possible [40]. However, the role of corticosteroids is controversial. There are both adverse [10, 68, 69] and positive reports [36, 37] with regard to their effects on CIP/CIM. ICU patients experiencing septic shock, ARDS, and acute asthma gain benefit from corticosteroids [16]. The duration and severity of MOF, which is an important risk factor for CIP/CIM, can also be decreased by the administration of corticosteroids [70]. Nevertheless, corticosteroids are an independent risk factor for assisted ventilation prolongation [37]. Hough et al. [71], who showed no impact of moderate doses of methylprednisolone on ICU-acquired neuromyopathy incidence, suggested that although corticosteroids result in more serious adverse events of neuromyopathy, they do not increase the total number of overall cases with ICU-acquired neuromuscular disorders.
Strict glycemic control (glucose levels between 80 and 110 mg/dl) via insulin therapy in order to avoid hyperglycemia also seems to prevent CIP/CIM [16], as insulin presents anti-inflammatory effects [72], protects the endothelium [73], improves lipid metabolism, and is also an anabolic hormone [16]. The mechanical ventilation duration and the patients’ dependency on mechanical ventilation over 2 weeks are also reduced by intensive insulin therapy [36, 74]. According to van den Berghe et al. [56], tight glycemic control with insulin administration in critical ill patients reduced the percentage of neuromyopathy from 51.9 to 28.7 %. Moreover, intensive insulin therapy in patients staying in the ICU for at least 1 week reduced the prevalence of CIP/CIM from 49 to 25 % in the surgical ICU (SICU) (p < 0.0001) and from 51 to 39 % in the medical ICU (MICU) (p = 0.02) [36, 37, 56, 75]. Additionally, patients requiring mechanical ventilation for at least 2 weeks were decreased from 42 to 32 % in the SICU (p = 0.04) and from 47 to 35 % in the MICU (p = 0.01) [36, 37, 56, 75]. Remarkably, a recent study suggested that even corticosteroids have a protective role against neuromyopathy when intensive insulin therapy is applied [37].
Recently, conventional methods of deep sedation and immobility in mechanically ventilated patients are doubted, as they gain profit from their early mobilization, which is also feasible and safe [76–78]. The European Respiratory Society and European Society of Intensive Care Medicine Task Force on Physiotherapy for Critically Ill Patients suggest that critically ill patients should be mobilized early [79]. Critical ill patients, who are early mobilized, get out of bed faster, stay less time in the hospital [80], are more often functionally independent after hospital discharge, are more likely to go home directly after hospitalization, and achieve better maximal walking distances while in the hospital as well as more ventilator-free days [81]. Rare adverse events are noticed after early mobilization [77, 80, 81]. Females, less sick patients (lower APACHE II score), patients who do not receive sedation and who are treated in the respiratory ICU are more likely to ambulate early [82]. On the other hand, deep sedation, respiratory and hemodynamic instability, brain injury, severe delirium or dementia, terminal diagnosis, spine or limb injury, CPR on admission and preexisting severe physical handicaps are criteria that suggest a patient’s exclusion from early mobilization [76]. Therefore, Hanekom et al. [78] recommended that a mobilization plan, depending on the patient’s tolerance for exercise and safety, should be programmed for each patient admitted to an ICU in order to achieve the patient’s early mobilization.
Electrical muscle stimulation (EMS), which is an alternative to active exercise [83], was reported to have a positive impact on muscle mass and strength preservation in critically ill patients [83–85]. EMS does not depend on patient cooperation, so it can be easily applied immediately after ICU admission [83, 85]. Catabolic effects of critical illness and immobilization to the muscle are reversed by the anabolic stimulus triggered by EMS [83–85]. EMS activates anti-inflammatory effects, improves microcirculation and mitochondrial function, promotes glucose oxidation, and the release antioxidant enzymes [83, 85]. Recent studies reported that not only does EMS lead to preserved muscle strength of directly stimulated muscle groups but it also results in muscle strength preservation of muscle groups not directly stimulated [83, 84]. Muscle mass of the stimulated muscle groups is also maintained [85]. Routsi et al. [83] suggested that 55-min EMS sessions per day in critically ill patients prevent CIP/CIM development and result in earlier weaning from mechanical ventilation and shorter ICU stay, thus decreasing morbidity. Therefore, EMS application is directly related to earlier rehabilitation and prevention of ICU-acquired weakness, as it promotes early mobilization, improves aerobic capacity, and shortens ICU and hospital stay [84].
Prognosis
Elevated mortality rates are reported in ICU patients experiencing CIP/CIM [5, 26, 33, 34], so diagnosis of electrophysiologic abnormalities at an early stage has prognostic value [32, 34]. The mortality rate in patients with CIP is as high as 60 % and is associated with underlying diseases rather than with CIP/CIM itself [3, 86]. However, such high mortality may be due to the underlying critical illness [30]. Apart from high mortality rates, CIP and CIM are associated with high morbidity rates due to increased ICU and hospital stays [26, 33] and due to prolonged rehabilitation and mechanical ventilation dependency [16]. Muscle weakness and paralysis are caused, thus affecting rehabilitation [34] in up to 100 % of patients staying in the ICU for over 4 weeks [53]. Moreover, assisted ventilation is required for 2–7 times more time in patients with critical illness neuromuscular disorders [12, 26, 31, 33], as the impairment of phrenic nerve and diaphragm is possible [12].
Over 50 % of patients completely recovered [12, 68, 87]. Most of them recovered after 4–12 weeks, but some patients have been reported to keep on suffering from weakness for at least 4 months [40]. Chronic disability is possible as clinical and neurophysiological signs may last for up to 5 years after ICU discharge [53, 88, 89]. Weeks are sufficient for clinical improvement in mild cases, while severe cases require months in order to improve in terms of physical disabilities [16]. In mild and moderate cases, rapid and full recovery, without any motor deficits or muscle weakness, is reported [12, 34, 53, 62, 68, 90, 91], whereas severe cases are related to incomplete recovery and high mortality [12, 62]. Tetraparesis, tetraplegia, or paraplegia is observed in up to 32 % of the most severely affected patients [68]. Even fully functionally recovered patients often suffer from persistent milder disabilities [16, 92]. According to Zifko et al. [91], six of 19 patients with CIP died in the first year, while 11 patients presented persistent neurological abnormalities impairing quality of life during 17 months after ICU discharge. In de Seze’s study [68], 11 of 19 patients with severe CIP fully recovered, whereas eight patients died or suffered from persistent weakness 2 years after hospital discharge. Prolonged duration of fever, prolonged ICU stay, and weight loss were associated with a poor recovery [68].
Conclusions
Critically ill ICU patients’ clinical course is often complicated by CIP and CIM [1]. Weaning is delayed, rehabilitation is impaired, hospital and ICU stay are prolonged, and mortality is increased because of CIP and CIM [16]. A distinction between these syndromes and other neuromuscular abnormalities beginning either before or after ICU admission is necessary [3]. Therapeutic measures aim at the prevention of CIP/CIM via preventing or facing the most important risk factors. Either prevention or aggressive treatment of sepsis can prevent CIP/CIM [2]. Limiting the dose and duration of NMBD administration is helpful in preventing prolonged neuromuscular blockade [2]. Shortening the duration of corticosteroid and NMBD administration limits the hazard of acute myopathy [2]. Furthermore, although the role of corticosteroids is controversial, the concurrent administration of corticosteroids and NMBDs should be avoided, unless the latter are necessary [40]. In addition, intensive insulin therapy reduces the incidence of CIP/CIM as well as the duration of ventilator support dependency [16, 74]. Finally, early mobilization, being absolutely feasible and safe, is beneficial for the patient’s rehabilitation [76–78]. Recent studies report that EMS, which is independent from patient’s cooperation, contributes in early mobilization and prevents ICU-acquired weakness [83–85].
References
Latronico N, Peli E, Botteri M. Critical illness myopathy and neuropathy. Curr Opin Crit Care. 2005;11:126–32.
Pandit L, Agrawal A. Neuromuscular disorders in critical illness. Clin Neurol Neurosurg. 2006;108:621–7.
Dhand UK. Clinical approach to the weak patient in the intensive care unit. Respir Care. 2006;51(9):1024–40.
Gorson KC, Ropper AH. Generalized paralysis in the intensive care unit: emphasis on the complications of neuromuscular blocking agents and corticosteroids. J Int Care Med. 1996;11:219–31.
De Jonghe B, Cook D, Sharshar T, Lefaucheur JP, Carlet J, Outin H. Acquired neuromuscular disorders in critically ill patients: a systematic review. Intensive Care Med. 1998;24:1242–50.
MacFarlane IA, Rosenthal FD. Severe myopathy after status asthmaticus (letter). Lancet. 1977;2(8038):615.
Bolton CF, Gilbert JJ, Hahn AF, Sibbald WJ. Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatry. 1984;47(11):1223–31.
Latronico N, Fenzi F, Recupero D, Guarneri B, Tomelleri G, Tonin P, De Maria G, Antonini L, Rizzuto N, Candiani A. Critical illness myopathy and neuropathy. Lancet. 1996;347:1579–82.
Tennila A, Salmi T, Pettila V, Roine RO, Varpula T, Takkunen O. Early signs of critical illness polyneuropathy in ICU patient with systemic inflammatory response syndrome or sepsis. Intensive Care Med. 2000;26:1360–3.
De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, Raphaël JC, Outin H, Bastuji-Garin S, Groupe de Réflexion et d’Etude des Neuromyopathies en Réanimation. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–67.
Zochodne DW, Bolton CF, Wells GA, Gilbert JJ, Hahn AF, Brown JD, Sibbald WA. Critical illness polyneuropathy: a complication of sepsis and multiple organ failure. Brain. 1987;110:819–41.
Witt NJ, Zochodne DW, Bolton CF, Grand’Maison F, Wells G, Young GB, Sibbald WJ. Peripheral nerve function in sepsis and multiple organ failure. Chest. 1991;99(1):176–84.
Bolton CF. Neuromuscular manifestations of critical illness. Muscle Nerve. 2005;32:140–63.
Fenzi F, Latronico N, Refatti N, Rizzuto N. Enhanced expression of E-selectin on the vascular endothelium of peripheral nerve in critically ill patients with neuromuscular disorders. Acta Neuropathol (Berl). 2003;106:75–82.
Hund E, Herkert M, Becker CM, Hacke W. A humoral neurotoxic factor in sera of patients with critical illness polyneuropathy. Ann Neurol. 1997;40:539.
Hermans G, De Jonghe B, Bruyninck F, Van den Berghe G. Clinical review: critical illness polyneuropathy and myopathy. Crit Care. 2008;12:238–46.
Bolton CF. Sepsis and the systemic inflammatory response syndrome: neuromuscular manifestations. Crit Care Med. 1996;24:1408–16.
Morisaki H, Sibbald WJ. Tissue oxygen delivery and the microcirculation. Crit Care Clin. 2004;20:213–23.
Bolton CF. Critical illness polyneuropathy. In: Thomas PK, Asbury A, editors. Peripheral nerve disorders II. Oxford: Butterworth-Heinemann; 1995. p. 262–80.
Di Giovanni S, Mirabella M, D’Amico A, Tonali P, Servidei S. Apoptotic features accompany acute quadriplegic myopathy. Neurology. 2000;55:854–8.
Essén P, McNurlan MA, Gamrin L, Hunter K, Calder G, Garlick PJ, Wernerman J. Tissue protein synthesis rates in critically ill patients. Crit Care Med. 1998;26:92–100.
Monk DN, Plank LD, Franch-Arcas G, Finn PJ, Streat SJ, Hill GL. Sequential changes in the metabolic response in critically patients during the first 25 days after blunt trauma. Surgery. 1996;223:395–405.
Rich MN, Pinter MJ, Kraner SD, Barchi RL. Loss of electrical excitability in an animal model of acute quadriplegic myopathy. Ann Neurol. 1998;43:171–9.
Rich MN, Pinter MJ. Sodium channel inactivation in an animal model of acute quadriplegic myopathy. Ann Neurol. 2001;50:26–33.
Rich MN, Pinter MJ. Crucial role of sodium channel fast inactivation in muscle fibre inexcitability in a rat model of critical illness myopathy. J Physiol. 2003;547:555–66.
Garnacho-Montero J, Madrazo-Osuna J, Garcia-Garmendia JL, Ortiz-Leyba C, Jiménez-Jiménez FJ, Barrero-Almodóvar A, Garnacho-Montero MC, Moyano-Del-Estad MR. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001;27:1288–96.
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–35.
Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33:1876–91.
Bercker S, Weber-Carstens S, Deja M, Grimm C, Wolf S, Behse F, Busch T, Falke KJ, Kaisers U. Critical illness polyneuropathy and myopathy in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33:711–5.
de Letter MA, Schmitz PI, Visser LH, Verheul FA, Schellens RL, Op de Coul DA, van der Meché FG. Risk factors for the development of polyneuropathy and myopathy in critically ill patients. Crit Care Med. 2001;29(12):2281–6.
De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30:1117–21.
Leijten FS, de Weerd AW, Poortvliet DC, De Ridder VA, Ulrich C, Harink-De Weerd JE. Critical illness polyneuropathy in multiple organ dysfunction syndrome and weaning from the ventilator. Intensive Care Med. 1996;22:856–61.
Garnacho-Montero J, Amaya-Villar R, Garcia-Garmendia JL, Madrazo-Osuna J, Ortiz-Leyba C. Effect of critical illness polyneuropathy on the withdrawal from mechanical ventilation and the length of stay in septic patients. Crit Care Med. 2005;33:349–54.
Leijten FS, Harinck-de Weerd JE, Poortvliet DC, de Weerd AW. The role of polyneuropathy in motor convalescence after prolonged mechanical ventilation. JAMA. 1995;274(15):1221–5.
Coakley JH, Nagendran K, Yarwood GD, Honavar M, Hinds CJ. Patterns of neurophysiological abnormality in prolonged critical illness. Intensive Care Med. 1998;24:801–7.
Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64:1348–53.
Hermans G, Wilmer A, Meersseman W, Milants I, Wouters PJ, Bobbaers H, Bruyninckx F, Van den Berghe G. Impact of intensive insulin therapy on neuromuscular complications and ventilator-dependency in MICU. Am J Respir Crit Care Med. 2007;175:480–9.
Apte-Kakade F. Rehabilitation of patients with quadriparesis after treatment of status asthmaticus with neuromuscular blocking agents and high dose corticosteroids. Arch Phys Med Rehabil. 1991;72:1024–8.
Gorson KC, Ropper AH. Acute respiratory failure neuropathy: a variant of critical illness polyneuropathy. Crit Care Med. 1993;21:267–71.
Gorson K. Approach to neuromuscular disorders in the intensive care unit. Neurocrit Care. 2005;03:195–212.
Tepper M, Rakic S, Haas JA, Woittiez AJ. Incidence and onset of critical illness polyneuropathy in patients with septic shock. Neth J Med. 2000;56:211–4.
Bednarik J, Lukas Z, Vondracek P. Critical illness polyneuromyopathy: the electrophysiological components of a complex entity. Intensive Care Med. 2003;29:1505–14.
Lacomis D, Giuliani MJ, Van Cott A, Kramer DJ. Acute myopathy of intensive care: clinical, electromyographic and pathological aspects. Ann Neurol. 1996;40:645–54.
Zifko UA, Zipko HT, Bolton CF. Clinical and electrophysiological findings in critical illness polyneuropathy. J Neurol Sci. 1998;159(2):186–93.
Maher J, Rutledge F, Remtulla H, Parkes A, Bernardi L, Bolton CF. Neuromuscular disorders associated with failure to wean from the ventilator. Intensive Care Med. 1995;21(9):737–43.
Bolton CF. Electrophysiologic studies of critically ill patients. Muscle Nerve. 1987;10(2):129–35.
Bolton CF. AAEM minimonograph #40: clinical neurophysiology of the respiratory system. Muscle Nerve. 1993;16(8):809–18.
Kimura J. Electrodiagnosis in diseases of nerve and muscle: principles and practice. 2nd ed. Philadelphia: FA Davis; 1989.
Latronico N, Rasulo FA. Presentation and management of ICU myopathy and neuropathy. Curr Opin Crit Care. 2010;16(2):123–7.
Stibler H, Edstrom L, Ahlbeck K, Remahl S, Ansved T. Electrophoretic determination of the myosin/actin ratio in the diagnosis of critical illness myopathy. Intensive Care Med. 2003;29(9):1515–27.
Lefaucheur JP, Nordine T, Rodriguez P, Brochard L. Origin of ICU acquired paresis determined by direct muscle stimulation. J Neurol Neurosurg Psychiatry. 2005;77:500–6.
Khan J, Burnham EL, Moss M. Acquired weakness in the ICU: critical illness myopathy and polyneuropathy. Minerva Anestesiol. 2006;72(6):401–6.
Fletcher SN, Kennedy DD, Ghosh IR, Misra VP, Kiff K, Coakley JH, Hinds CJ. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med. 2003;31:1012–6.
Segredo V, Caldwell JE, Matthay MA, Sharma ML, Gruenke LD, Miller RD. Persistent paralysis in critically ill patients after long-term administration of vecuronium. N Engl J Med. 1992;327:524–8.
Partridge BL, Abrams JH, Bazemore C, Rubin R. Prolonged neuromuscular blockade after long-term infusion of vecuronium bromide in the intensive care unit. Crit Care Med. 1990;18:1177–9.
Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67.
Khovidhunkit W, Memon RA, Feingold KR, Grunfeld C. Infection and inflammation-induced proatherogenic changes of lipoproteins. J Infect Dis. 2000;181:S462–72.
Mohr M, Englisch L, Roth A, Burchardi H, Zielmann S. Effects of early treatment with immunoglobulin on critical illness polyneuropathy following multiple organ failure and gram-negative sepsis. Intensive Care Med. 1997;23:1144–9.
Wijdicks EFM, Fulgham JR. Failure of high dose intravenous immunoglobulins to alter the clinical course of critical illness polyneuropathy. Muscle Nerve. 1994;17:1494–5.
Martin UJ, Hincapie L, Nimchuk M, Gaughan J, Criner GJ. Impact of whole-body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–65.
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–56.
Bolton CF, Laverty DA, Brown JD, Witt NJ, Hahn AF, Sibbald WJ. Critically ill polyneuropathy: electrophysiological studies and differentiation from Guillain–Barré syndrome. J Neurol Neurosurg Psychiatry. 1986;49:563–73.
Berard MP, Zazzo JF, Condat P, Vasson MP, Cynober L. Total parenteral nutrition enriched with arginine and glutamate generates glutamine and limits protein catabolism in surgical patients hospitalized in intensive care units. Crit Care Med. 2000;28:3637–44.
Yu YM, Ryan CM, Fei ZW, Lu XM, Castillo L, Schultz JT, Tompkins RG, Young VR. Plasma L-5-oxoproline kinetics and whole blood glutathione synthesis rates in severely burned adult humans. Am J Physiol Endocrinol Metab. 2002;282:E247–58.
Schols AM, Soeters PB, Mostert R, Pluymers RJ, Wouters EF. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trial. Am J Respir Crit Care Med. 1995;152:1268–74.
Pichard C, Kyle U, Chevrolet JC, Jolliet P, Slosman D, Mensi N, Temler E, Ricou B. Lack of effects of recombinant growth hormone on muscle function in patients requiring prolonged mechanical ventilation: a prospective, randomized, controlled study. Crit Care Med. 1996;24:403–13.
Leatherman JW, Fluegel WL, David WS, Davies SF, Iber C. Muscle weakness in mechanically ventilated patients with severe asthma. Am J Respir Crit Care Med. 1996;153:1686–90.
de Seze M, Petit H, Wiart L, Cardinaud JP, Gaujard E, Joseph PA, Mazaux JM, Barat M. Critical illness polyneuropathy. A 2-year follow-up study in 19 severe cases. Eur Neurol. 2000;43:61–9.
Campellone JV, Lacomis D, Kramer DJ, Van Cott AC, Giuliani MJ. Acute myopathy after liver transplantation. Neurology. 1998;50:46–53.
Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for treating severe sepsis and septic shock. Cochrane Database Syst Rev 2004;(1):CD002243.
Hough CL, Steinberg KP, Taylor Thompson B, Rubenfeld GD, Hudson LD. Intensive care unit-acquired neuromyopathy and corticosteroids in survivors of persistent ARDS. Intensive Care Med. 2009;35(1):63–8.
Hansen TK, Thiel S, Wouters PJ, Christiansen JS, Van den Berghe G. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab. 2003;88:1082–8.
Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, Hansen TK, Van den Berghe G. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest. 2005;115:2277–86.
Hermans G, Schrooten M, Van Damme P, Berends N, Bouckaert B, De Vooght W, Robberecht W, Van den Berghe G. Benefits of intensive insulin therapy on neuromuscular complications in routine daily critical care practice: a retrospective study. Crit Care. 2009;13(1):R5.
Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61.
Schweickert WD, Kress JP. Implementing early mobilization interventions in mechanically ventilated patients in the ICU. Chest. 2011;140(6):1612–7.
Bailey P, Thomsen GE, Spuhler VJ, Blair R, Jewkes J, Bezdjian L, Veale K, Rodriquez L, Hopkins RO. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–45.
Hanekom S, Gosselink R, Dean E, van Aswegen H, Roos R, Ambrosino N, Louw Q. The development of a clinical management algorithm for early physical activity and mobilization of critically ill patients: synthesis of evidence and expert opinion and its translation into practice. Clin Rehabil. 2011;25(9):771–87.
Gosselink R, Bott J, Johnson M, Dean E, Nava S, Norrenberg M, Schönhofer B, Stiller K, van de Leur H, Vincent JL. Physiotherapy for adult patients with critical illness: recommendations of the European Respiratory Society and European Society of Intensive Care Medicine Task Force on Physiotherapy for Critically Ill patients. Intensive Care Med. 2008;34:1188–99.
Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, Ross A, Anderson L, Baker S, Sanchez M, Penley L, Howard A, Dixon L, Leach S, Small R, Hite RD, Haponik E. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–43.
Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister KE, Hall JB, Kress JP. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–82.
Thomsen GE, Snow GL, Rodriguez L, Hopkins RO. Patients with respiratory failure increase ambulation after transfer to an intensive care unit where early activity is a priority. Crit Care Med. 2008;36(4):1119–24.
Routsi C, Gerovasili V, Vasileiadis I, Karatzanos E, Pitsolis T, Tripodaki E, Markaki V, Zervakis D, Nanas S. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care. 2010;14(2):R74.
Karatzanos E, Gerovasili V, Zervakis D, Tripodaki ES, Apostolou K, Vasileiadis I, Papadopoulos E, Mitsiou G, Tsimpouki D, Routsi C, Nanas S. Electrical muscle stimulation: an effective form of exercise and early mobilization to preserve muscle strength in critically ill patients. Crit Care Res Pract. 2012;2012:432752.
Gerovasili V, Stefanidis K, Vitzilaios K, Karatzanos E, Politis P, Koroneos A, Chatzimichail A, Routsi C, Roussos C, Nanas S. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care. 2009;13(5):R161.
Magistris MR. Critical illness neuropathies. Rev Neurol (Paris). 2002;158:293–9.
Latronico N, Shehu I, Seghelini E. Neuromuscular sequelae of critical illness. Curr Opin Crit Care. 2005;11:381–90.
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS, Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–93.
Leijten FS. Survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(21):2149–50.
Bolton CF. The polyneuropathy of critical illness. J Intensive Care Med. 1994;9:132–8.
Zifko UA. Long-term outcome of critical illness polyneuropathy. Muscle Nerve Suppl. 2000;9:S49–52.
van der Schaaf M, Beelen A, de Vos R. Functional outcome in patients with critical illness polyneuropathy. Disabil Rehabil. 2004;26:1189–97.
Conflict of interest
No conflicts of interest are declared and no financial support was received for this work.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Apostolakis, E., Papakonstantinou, N.A., Baikoussis, N.G. et al. Intensive care unit-related generalized neuromuscular weakness due to critical illness polyneuropathy/myopathy in critically ill patients. J Anesth 29, 112–121 (2015). https://doi.org/10.1007/s00540-014-1875-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-014-1875-x