Abstract
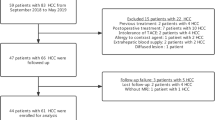
Induction chemotherapy with docetaxel improved outcome in advanced head and neck squamous cell carcinoma (HNSCC) patients, but docetaxel was not recommended in liver dysfunction patients for treatment toxicities. Severe neutropenic events (SNE) including severe neutropenia (SN) and febrile neutropenia (FN) still developed in these patients with normal serum transaminases. Ultrasonography (US) fibrotic score represented degree of hepatic parenchymal damage and showed good correlation to fibrotic changes histologically. This study aims to evaluate the association of US fibrotic score with docetaxel treatment-related SNE in advanced HNSCC patients with normal serum transaminases. Between 1 January 2011 and 31 December 2013, a total of 47 advanced HNSCC patients treated with induction docetaxel were enrolled. The clinical features were collected to assess predictive factors for SNE. The patients were divided into two groups by the US fibrotic score with a cutoff value of 7. The Mann–Whitney U test and logistic regression method were used for the risk factor analysis. The background, treatment, and response were similar in both groups except for lower lymphocyte and platelet count in patients with higher US score. Twenty-seven patients (51 %) developed grade 3/4 neutropenia, and more SNE developed in patients with US score ≧7. In multivariate analysis, only US score ≥7 was independent predictive factor for developing SN (hazard ratio 7.71, p = 0.043) and FN (hazard ratio 20.95, p = 0.008). US score ≥7 is an independent risk factor for SNE in advanced HNSCC patients treated with induction docetaxel. US score could be used for risk prediction of docetaxel-related SNE.
Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Department of Health T, Republic of China, Cancer registration report. DOH Report 2012 2012
Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M et al (2007) Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 357(17):1695–1704
Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V et al (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357(17):1705–1715
Bruno R, Vivier N, Vergniol JC, De Phillips SL, Montay G, Sheiner LB (1996) A population pharmacokinetic model for docetaxel (Taxotere): model building and validation. J Pharmacokinet Biopharm 24(2):153–172
Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT et al (1998) Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 16(1):187–196
Bruno R, Vivier N, Veyrat-Follet C, Montay G, Rhodes GR (2001) Population pharmacokinetics and pharmacokinetic-pharmacodynamic relationships for docetaxel. Investig New Drugs 19(2):163–169
Minami H, Kawada K, Sasaki Y, Tahara M, Igarashi T, Itoh K et al (2009) Population pharmacokinetics of docetaxel in patients with hepatic dysfunction treated in an oncology practice. Cancer Sci 100(1):144–149
Francis PBR, Seidman A et al (1994) Pharmacodynamics (PD) of docetaxel (Taxotere®) in patients (PTS) with liver metastases (METS). Proc Am Soc Clin Oncol 13:138
Lin DY, Sheen IS, Chiu CT, Lin SM, Kuo YC, Liaw YF (1993) Ultrasonographic changes of early liver cirrhosis in chronic hepatitis B: a longitudinal study. J Clin Ultrasound 21(5):303–308
Hung CH, Lu SN, Wang JH, Lee CM, Chen TM, Tung HD et al (2003) Correlation between ultrasonographic and pathologic diagnoses of hepatitis B and C virus-related cirrhosis. J Gastroenterol 38(2):153–157
Institute NC (2009) Common terminology criteria for adverse events v4.0. NCI, NIH, DHHS
Hitt R, Lopez-Pousa A, Martinez-Trufero J, Escrig V, Carles J, Rizo A et al (2005) Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol 23(34):8636–8645
Paccagnella A, Ghi MG, Loreggian L, Buffoli A, Koussis H, Mione CA et al (2010) Concomitant chemoradiotherapy versus induction docetaxel, cisplatin and 5 fluorouracil (TPF) followed by concomitant chemoradiotherapy in locally advanced head and neck cancer: a phase II randomized study. Ann Oncol 21(7):1515–1522
Pointreau Y, Garaud P, Chapet S, Sire C, Tuchais C, Tortochaux J et al (2009) Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst 101(7):498–506
Millward MJ, Boyer MJ, Lehnert M, Clarke S, Rischin D, Goh BC et al (2003) Docetaxel and carboplatin is an active regimen in advanced non-small-cell lung cancer: a phase II study in Caucasian and Asian patients. Ann Oncol 14(3):449–454
Yano R, Konno A, Watanabe K, Tsukamoto H, Kayano Y, Ohnaka H et al (2013) Pharmacoethnicity of docetaxel-induced severe neutropenia: integrated analysis of published phase II and III trials. Int J Clin Oncol 18(1):96–104
Watanabe M, Nagai Y, Kinoshita K, Saito S, Kurashige J, Karashima R et al (2011) Induction chemotherapy with docetaxel/cisplatin/5-fluorouracil for patients with node-positive esophageal cancer. Digestion 83(3):146–152
Kang YK, Ryu MH, Yoo C, Chang HM, Yook JH, Oh ST et al (2011) Phase I/II study of a combination of docetaxel, capecitabine, and cisplatin (DXP) as first-line chemotherapy in patients with advanced gastric cancer. Cancer Chemother Pharmacol 67(6):1435–1443
Lu YS, Chen DR, Tseng LM, Yeh DC, Chen ST, Hsieh CM et al (2011) Phase II study of docetaxel, capecitabine, and cisplatin as neoadjuvant chemotherapy for locally advanced breast cancer. Cancer Chemother Pharmacol 67(6):1257–1263
Katori H, Tsukuda M (2005) Comparison of induction chemotherapy with docetaxel, cisplatin, and 5-fluorouracil (TPF) followed by radiation vs concurrent chemoradiotherapy with TPF in patients with locally advanced squamous cell carcinoma of the head and neck. Clin Oncol 17(3):148–152
Chang YF, Lo AC, Tsai CH, Lee PY, Sun S, Chang TH et al (2013) Higher complication risk of totally implantable venous access port systems in patients with advanced cancer—a single institution retrospective analysis. Palliat Med 27(2):185–191
Nieuweboer AJ, de Morree ES, de Graan AJ, Sparreboom A, de Wit R, Mathijssen RH (2015) Inter-patient variability in docetaxel pharmacokinetics: a review. Cancer Treat Rev 41(7):605–613
Baker SD, Li J, ten Tije AJ, Figg WD, Graveland W, Verweij J et al (2005) Relationship of systemic exposure to unbound docetaxel and neutropenia. Clin Pharmacol Ther 77(1):43–53
Puisset F, Alexandre J, Treluyer JM, Raoul V, Roche H, Goldwasser F et al (2007) Clinical pharmacodynamic factors in docetaxel toxicity. Br J Cancer 97(3):290–296
Kacevska M, Robertson GR, Clarke SJ, Liddle C (2008) Inflammation and CYP3A4-mediated drug metabolism in advanced cancer: impact and implications for chemotherapeutic drug dosing. Expert Opin Drug Metab Toxicol 4(2):137–149
Baker SD, Verweij J, Cusatis GA, van Schaik RH, Marsh S, Orwick SJ et al (2009) Pharmacogenetic pathway analysis of docetaxel elimination. Clin Pharmacol Ther 85(2):155–163
Tran A, Jullien V, Alexandre J, Rey E, Rabillon F, Girre V et al (2006) Pharmacokinetics and toxicity of docetaxel: role of CYP3A, MDR1, and GST polymorphisms. Clin Pharmacol Ther 79(6):570–580
Lewis LD, Miller AA, Owzar K, Bies RR, Markova S, Jiang C et al (2013) The relationship of polymorphisms in ABCC2 and SLCO1B3 with docetaxel pharmacokinetics and neutropenia: CALGB 60805 (Alliance). Pharmacogenet Genomics 23(1):29–33
Felici A, Loos WJ, Verweij J, Cirillo I, de Bruijn P, Nooter K et al (2006) A pharmacokinetic interaction study of docetaxel and cisplatin plus or minus 5-fluorouracil in the treatment of patients with recurrent or metastatic solid tumors. Cancer Chemother Pharmacol 58(5):673–680
Marre F, Sanderink GJ, de Sousa G, Gaillard C, Martinet M, Rahmani R (1996) Hepatic biotransformation of docetaxel (Taxotere) in vitro: involvement of the CYP3A subfamily in humans. Cancer Res 56(6):1296–1302
van Zuylen L, Verweij J, Nooter K, Brouwer E, Stoter G, Sparreboom A (2000) Role of intestinal P-glycoprotein in the plasma and fecal disposition of docetaxel in humans. Clin Cancer Res 6(7):2598–2603
Hooker AC, Ten Tije AJ, Carducci MA, Weber J, Garrett-Mayer E, Gelderblom H et al (2008) Population pharmacokinetic model for docetaxel in patients with varying degrees of liver function: incorporating cytochrome P4503A activity measurements. Clin Pharmacol Ther 84(1):111–118
Boyer JL (1976) Chronic hepatitis—a perspective on classification and determinants of prognosis. Gastroenterology 70(6):1161–1171
Mehanna H, Paleri V, West CML, Nutting C (2010) Head and neck cancer—part 1: epidemiology, presentation, and prevention. BMJ 341:c4684
Purdue MP, Hashibe M, Berthiller J, La Vecchia C, Dal Maso L, Herrero R et al (2009) Type of alcoholic beverage and risk of head and neck cancer—a pooled analysis within the INHANCE Consortium. Am J Epidemiol 169(2):132–142
Sasco AJ, Secretan MB, Straif K (2004) Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer 45(Suppl 2):S3–S9
Zwiebel WJ (1995) Sonographic diagnosis of diffuse liver disease. Semin Ultrasound CT MR 16(1):8–15
Afzal S, Masroor I, Beg M (2013) Evaluation of chronic liver disease: does ultrasound scoring criteria help? Int J Chronic Dis 2013:326231
Shiota M, Yokomizo A, Takeuchi A, Kiyoshima K, Inokuchi J, Tatsugami K et al (2014) Risk factors for febrile neutropenia in patients receiving docetaxel chemotherapy for castration-resistant prostate cancer. Support Care Cancer 22(12):3219–3226
Shigeta K, Kosaka T, Yazawa S, Yasumizu Y, Mizuno R, Nagata H et al (2015) Predictive factors for severe and febrile neutropenia during docetaxel chemotherapy for castration-resistant prostate cancer. Int J Clin Oncol 20(3):605–612
Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A et al (2014) Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 106(6):dju124
Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ (2013) The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 88(1):218–230
Foucher J, Chanteloup E, Vergniol J, Castera L, Le Bail B, Adhoute X et al (2006) Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 55(3):403–408
Acknowledgments
The authors thank the Office of Medical Records and the Office of Medical Information, Chang-Gung Memorial Hospital, for providing the data of the patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests. The authors have full control of all primary data and agree to allow the journal to review our data if requested.
Rights and permissions
About this article
Cite this article
Wang, TY., Chen, WM., Yang, LY. et al. Score of liver ultrasonography predicts treatment-related severe neutropenia and neutropenic fever in induction chemotherapy with docetaxel for locally advanced head and neck cancer patients with normal serum transamines. Support Care Cancer 24, 4697–4703 (2016). https://doi.org/10.1007/s00520-016-3318-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3318-8