Abstract
Background
This study was conducted to determine the optimal dosage of the docetaxel-capecitabine-cisplatin (DXP) regimen and to evaluate its efficacy and safety in patients with advanced gastric cancer.
Methods
Patients with advanced gastric or esophagogastric junctional adenocarcinoma received capecitabine (days 1–14) and intravenous docetaxel and cisplatin (day 1) every 3 weeks.
Results
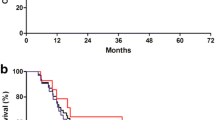
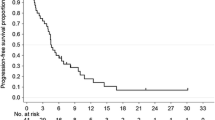
In the phase I study, 15 patients were treated with 4 different dose levels. Asthenia and neutropenic fever were the dose-limiting toxicities. For the phase II study, 1,125 mg/m2 of capecitabine was initially recommended with 60 mg/m2 docetaxel and 60 mg/m2 cisplatin. However, frequent dose modifications at this dose level resulted in a final optimal dose of 937.5 mg/m2 capecitabine. Among the 40 patients enrolled in the phase II study, 4 complete and 23 partial responses were observed, presenting objective response rate of 68%. Ten patients achieving good response with complete disappearance of distant metastases underwent surgery, and 4 pathologic complete responses were identified. After the median follow-up of 83.7 months (range, 20.2–86.5) in surviving patients, the median overall survival was 14.4 months and median progression-free survival was 7.6 months. The most frequent grade 3/4 adverse events were neutropenia (62.5%) and asthenia (37.5%). Ten per cent of the patients experienced neutropenic fever, with one case of sepsis-induced death.
Conclusion
DXP displays considerable antitumor activity, and may thus present effective first-line treatment for advanced gastric cancer. Further investigation of the efficacy and safety of this regimen in both first-line and neoadjuvant settings is warranted.


Similar content being viewed by others
References
Terry MB, Gaudet MM, Gammon MD (2002) The epidemiology of gastric cancer. Semin Radiat Oncol 12:111–127
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Ajani JA, Takiuchi H (1999) Recent developments in oral chemotherapy options for gastric carcinoma. Drugs 58(Suppl 3):85–90
Chang JC (1994) Perspectives on stomach cancer. J Korean Med Sci 9:277–280
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE (2006) Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24:2903–2909
Lacave AJ, Baron FJ, Anton LM, Estrada E, De Sande LM, Palacio I et al (1991) Combination chemotherapy with cisplatin and 5-fluorouracil 5-day infusion in the therapy of advanced gastric cancer: a phase II trial. Ann Oncol 2:751–754
Rougier P, Ducreux M, Mahjoubi M, Pignon JP, Bellefqih S, Oliveira J et al (1994) Efficacy of combined 5-fluorouracil and cisplatinum in advanced gastric carcinomas. A phase II trial with prognostic factor analysis. Eur J Cancer 30A:1263–1269
Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC et al (1993) A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer 71:3813–3818
Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E et al (2000) Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol 18:2648–2657
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24:4991–4997
Hong YS, Song SY, Lee SI, Chung HC, Choi SH, Noh SH et al (2004) A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol 15:1344–1347
Koizumi W, Saigenji K, Ujiie S, Terashima M, Sakata Y, Taguchi T (2003) A pilot phase II study of capecitabine in advanced or recurrent gastric cancer. Oncology 64:232–236
Sakamoto J, Chin K, Kondo K, Kojima H, Terashima M, Yamamura Y et al (2006) Phase II study of a 4-week capecitabine regimen in advanced or recurrent gastric cancer. Anticancer Drugs 17:231–236
Kim TW, Kang YK, Ahn JH, Chang HM, Yook JH, Oh ST et al (2002) Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced gastric cancer. Ann Oncol 13:1893–1898
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Fleming TR (1982) One-sample multiple testing procedure for phase II clinical trials. Biometrics 38:143–151
Simon R (1987) How large should a phase II trial of a new drug be? Cancer Treat Rep 71:1079–1085
Ishitsuka H (2000) Capecitabine: preclinical pharmacology studies. Invest New Drugs 18:343–354
Park YH, Ryoo BY, Choi SJ, Kim HT (2004) A phase II study of capecitabine and docetaxel combination chemotherapy in patients with advanced gastric cancer. Br J Cancer 90:1329–1333
Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T et al (2004) XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol 22:2084–2091
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36–46
Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J et al (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20:666–673
Okines AF, Norman AR, McCloud P, Kang YK, Cunningham D (2009) Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol 20:1529–1534
Lorenzen S, Hentrich M, Haberl C, Heinemann V, Schuster T, Seroneit T et al (2007) Split-dose docetaxel, cisplatin and leucovorin/fluorouracil as first-line therapy in advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: results of a phase II trial. Ann Oncol 18:1673–1679
Roth AD, Fazio N, Stupp R, Falk S, Bernhard J, Saletti P et al (2007) Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol 25:3217–3223
Sym SJ, Ryu MH, Kang HJ, Lee SS, Chang HM, Lee JL et al (2010) Phase I study of 3-weekly docetaxel, capecitabine and oxaliplatin combination chemotherapy in patients with previously untreated advanced gastric cancer. Cancer Chemother Pharmacol 66:373–380
Zang DY, Yang DH, Kim MJ, Jang KM, Hwang SW, Yoo KS et al (2009) Dose-finding study of docetaxel, oxaliplatin, and S-1 for patients with advanced gastric cancer. Cancer Chemother Pharmacol 64:877–883
Sym SJ, Chang HM, Ryu MH, Lee JL, Kim TW, Yook JH et al (2010) Neoadjuvant docetaxel, capecitabine and cisplatin (DXP) in patients with unresectable locally advanced or metastatic gastric cancer. Ann Surg Oncol 17:1024–1032
Park IH, Kim SY, Kim YW, Ryu KW, Lee JH, Lee JS et al (2010) Clinical characteristics and treatment outcomes of gastric cancer patients with isolated para-aortic lymph node involvement. Cancer Chemother Pharmacol. doi:10.1007/s00280-010-1296-y
Lee JL, Ryu MH, Chang HM, Kim TW, Yook JH, Oh ST et al (2008) A phase II study of docetaxel as salvage chemotherapy in advanced gastric cancer after failure of fluoropyrimidine and platinum combination chemotherapy. Cancer Chemother Pharmacol 61:631–637
Moon YW, Rha SY, Jeung HC, Kim C, Hong MH, Chang H et al (2010) Outcomes of multiple salvage chemotherapy for advanced gastric cancer: implications for clinical practice and trial design. Cancer Chemother Pharmacol. doi:10.1007/s00280-010-1295-z
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M et al (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Van Cutsem E, Kang Y, Chung H, Shen L, Sawaki A, Lordick F et al (2009) Efficacy results from the ToGA trial: a phase III study of trastuzumab added to standard chemotherapy (CT) in first-line human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (GC). J Clin Oncol 27: Abstract LBA4509
Kang YK, Yoon DH, Ryoo BY, Ryu MH (2010) Recent advances in chemotherapy for advanced gastric cancer. APJOH 2(1):67–74
Lee JL, Kang HJ, Kang YK, Ryu MH, Chang HM, Kim TW et al (2008) Phase I/II study of 3-week combination of S-1 and cisplatin chemotherapy for metastatic or recurrent gastric cancer. Cancer Chemother Pharmacol 61:837–845
Conflict of interest
Yoon-Koo Kang: Consultant for Roche and Sanofi-Aventis and honoraria for Roche and Sanofi-Aventis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, YK., Ryu, MH., Yoo, C. et al. Phase I/II study of a combination of docetaxel, capecitabine, and cisplatin (DXP) as first-line chemotherapy in patients with advanced gastric cancer. Cancer Chemother Pharmacol 67, 1435–1443 (2011). https://doi.org/10.1007/s00280-010-1444-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1444-4