Abstract
Introduction
Upper intestinal leaks and perforations are associated with high morbidity and mortality rates. Despite the growing experience using endoscopically placed stents, the treatment of these leaks and perforations remain a challenge. Endoluminal vacuum (E-Vac) therapy is a novel treatment that has been successfully used in Germany to treat upper gastrointestinal leaks and perforations. There currently are no reports on its use in the USA.
Methods
E-Vac therapy was used to treat 11 patients with upper gastrointestinal leaks and perforations from September 2013 to September 2014. Five patients with leaks following sleeve gastrectomy were excluded from this study. A total of six patients were treated with E-Vac therapy; these included: (n = 2) iatrogenic esophageal perforations, (n = 1) iatrogenic esophageal and gastric perforations, (n = 1) iatrogenic gastric perforation, (n = 1) gastric staple line leak following a surgical repair of a traumatic gastric perforation, and (n = 1) esophageal perforation due to an invasive fungal infection. Four patients had failed an initial surgical repair prior to starting E-Vac therapy.
Results
All six patients (100 %) had complete closure of their perforation or leak after an average of 35.8 days of E-Vac therapy requiring 7.2 different E-Vac changes. No deaths occurred in the 30 days following E-Vac therapy. One patient died following complete closure of his perforation and transfer to an acute care facility due to an unrelated complication. There were no complications directly related to the use of E-Vac therapy. Only one patient had any symptoms of dysphagia. This patient had severe dysphagia from an esophagogastric anastomotic stricture prior to her iatrogenic perforations. Following E-Vac therapy, her dysphagia had actually improved and she could now tolerate a soft diet.
Conclusions
E-Vac therapy is a promising new method in the treatment of upper gastrointestinal leaks and perforations. Current successes need to be validated through future prospective controlled studies.
Similar content being viewed by others
Laparoscopic foregut surgery is associated with a low risk (1–3.3 %) of an iatrogenic perforation of the stomach or esophagus [1–3]. However, the risk of perforation can increase significantly (14.6–31.2 %) in re-operative cases [3]. Most perforations are recognized and closed intra-operatively with minimal morbidity and mortality [3]. Patient morbidity and mortality significantly increase, though, when iatrogenic perforations are discovered and treated in the post-operative period [3].
Esophageal perforations are a life-threatening, morbid condition associated with a 13.2 % mortality rate [4]. Death is the end result to what starts as a robust local and systemic inflammatory response to mediastinal contamination. Progression to sepsis and septic shock can quickly occur in the absence of prompt and effective drainage and removal of the septic focus [4]. Delays in treatment for more than 24 h are associated with a threefold increase in mortality rates [4].
An aggressive surgical approach is believed to be the most effective way to remove the septic focus and prevent further contamination [4, 5]. Despite its perceived advantage surgical therapy continues to be associated with high mortality rates [4, 5]. There has also been a growing interest in the use of covered stents to treat esophageal perforations. Results from initial studies were promising but consisted of small numbers of highly selected patients. Lately a number of treatment failures have been reported. These failures have highlighted a number of key factors that significantly reduce the effectiveness of stents [6]. One key limiting factor with stent placement is the additional need for surgical interventions to provide drainage and removal of the septic focus [7].
Endoluminal vacuum (E-Vac) therapy uses the same treatment principles seen with vacuum-assisted closure therapy of external wounds. Both improve and accelerate healing by removing infected secretions, reducing edema, increasing local perfusion, and promoting granulation tissue formation [8, 9]. E-Vac therapy has been shown to successfully close esophageal perforations and leaks with low mortality rates [8–15]. All reported studies have come from German institutions. To our knowledge there have been no reported studies on the use of E-Vac therapy here in the USA. This report summarizes our initial experience using E-Vac therapy to treat upper gastrointestinal leaks and perforations.
Materials and methods
Based upon evidence from prior studies we began using E-Vac therapy at our own institution in 2013 to treat upper gastrointestinal leaks and perforations in patients who had failed other therapies or were poor surgical or stent candidates. In this retrospective study we identified 11 patients with upper gastrointestinal leaks and perforations that were treated with E-Vac within the first year (September 17, 2013 and September 17, 2014) of adopting this technique. Five patients with leaks following sleeve gastrectomy were excluded due to their unique pathology and other management needs in addition to closure of the leak or perforation (all had complete closure of their leak with E-Vac therapy). The remaining six patients had perforations or leaks involving the esophagus and/or stomach and were included in this study.
For the type and etiology of the perforation or leak see Table 1. In patient #1 an esophageal perforation occurred during endoscopic balloon dilation for achalasia. Patient #2 had an esophageal perforation that occurred during a paraesophageal hernia repair. Patient #3 had a severely torn esophagogastric anastomosis and three different gastric perforations during an attempted repair of a paraesophageal hernia. Patient #4 had a recurrent leak at the gastric staple line of a repaired traumatic gastric perforation. Patient #5 had an esophageal perforation due to an invasive fungal infection. Patient #6 had a gastric perforation that occurred during a re-do Nissen fundoplication. All patients were managed in a multidisciplinary fashion that included a gastroenterologist, cardiothoracic surgeon, and/or general surgeon. When in the ICU, critical care management was provided by our trauma/acute care surgeons.
Clinical data including vital signs and laboratory data were collected in all patients. Hospital length of stay and length of stay in the intensive care unit (ICU) were recorded from when E-Vac therapy was started. Duration of E-Vac therapy, number of foam changes, and days between each foam change were recorded for each patient. Time to complete closure of the perforation or leak was recorded from the start of E-Vac therapy. Complete closure of the perforation or leak was defined as a completely sealed cavity of 1 cm or less in depth that did not require any further treatment in the follow-up period. A completely sealed cavity was determined on endoscopy if granulation tissue was present at the base of the cavity and no tunneling or fistula tracts could be identified. Complete sealing was also confirmed by the absence of contrast extravasation on either a CT with oral contrast, an esophagogram, or an upper gastrointestinal (GI) study.
Patient comorbidities
A list of significant patient comorbidities is given in Table 1. On average the patients were 60.2 years of age with a male to female ratio of 2:4. All patients had an American Society of Anesthesiology (ASA) Physical Status Classification of IV except patient #4 (ASA III). Patients had an average Charlson Comorbidity Index (CCI) age adjusted score of 3.8 (range 0–6). Three patients developed significant complications prior to the start of E-Vac therapy including pulmonary embolus (n = 2) and respiratory failure (n = 3).
The 30-day in-hospital risk of death (Table 3) was calculated for each patient using the American College of Surgeons National Surgical Quality Improvement Project (ACS-NSQIP) surgical risk calculator. All variables were identified by reviewing the patient’s chart. In four patients the type of operation used in the calculator was based upon the operation they received to repair their perforation. In the two patients who were initially treated with E-Vac therapy the surgical therapy associated with the lowest morbidity was chosen (patient #1, laparoscopic Heller myotomy with fundoplasty, CPT code 43279, patient #5, suture of esophageal wound or injury, CPT 43415). The average predicted 30-day in-hospital mortality rate of all patients was 26 % (3–60 %).
Disease severity
Specific disease severity indicators for each patient are given in Table 1. All patients had complete transmural perforations of the esophagus and/or stomach. Four patients (patients #1, 2, 3, and 5) had perforations with mediastinal and pleural extension. Two patients (patient #4 and 5) with recurrent leaks from their repaired gastric perforations had focal peritonitis prior to starting E-Vac therapy.
All patients met NSQIP criteria for sepsis with two patients having septic shock requiring vasopressor therapy. In five patients E-Vac therapy was started greater than 24 h after symptom onset (patient #5) or diagnosis (patients #2, 3, 4, and 6). In patient #1 E-Vac therapy was started within 6 h following her endoscopic balloon dilation procedure. E-Vac therapy was the initial treatment method used in two patients (patient #1 and 5). Patient #2 had delayed recognition of her esophageal perforation that was treated with a thoracotomy and primary repair followed by stent placement. Patient #3 had an intra-operative repair of her iatrogenic perforations. In patient #4 a gastric perforation was discovered 5 days following an exploratory laparotomy and was treated with an abbreviated laparotomy and a stapled repair. Final abdominal closure occurred on his fourth abdominal washout. Patient #6 was treated with a laparotomy and primary repair with an omental patch. All initial perforations and recurrent leaks were diagnosed through the use of imaging (esophagram, upper GI, or CT with oral contrast) and confirmed on endoscopy prior to starting E-Vac therapy.
E-Vac therapy
The Endo-Sponge™ (B Braun Melsungen AG, Melsungen, Germany) is the only commercially made device for E-Vac therapy and is currently only FDA approved in the treatment of anastomotic leaks involving the rectum. We, in addition to other authors, have adapted the current Wound V.A.C.™ (Lifecell, Bridgewater, NJ) device for use in performing E-Vac therapy.
Endoscopic exam
E-Vac therapy was performed in all patients under general endotracheal anesthesia. The majority of E-Vac therapy procedures were performed in the gastrointestinal suite. In two patients (patients #3 and 5) the initial placement of E-Vac therapy occurred in the operating room due to the possible need for surgical intervention. In three patients who were mechanically ventilated E-Vac therapy was initially placed (patient #2) or changed out (patient #3 and 4) at the bedside in the ICU.
An endoscopic examination was performed to confirm the defect and to endoscopically identify the presence of an associated cavity. If a cavity was present it was explored, irrigated, and debrided. Exploration of the mediastinum, chest, and abdomen can result in free air accumulation within these spaces. The amount of free air is usually minimal and limited to the first 1–2 endoscopies. However, a general awareness is needed to prevent unnecessary morbidity from what should be a fairly benign complication. In our experience this complication has not required any intervention other than close monitoring of the patient and having a chest X-ray performed immediately following endoscopy. If already in place, chest tubes should be placed to suction during the procedure and kept to suction until a chest X-ray can confirm the absence of a large pneumothorax. The consequences of free air accumulation can also be mitigated through the use of carbon dioxide insufflation.
Placement
After a thorough endoscopic exam a nasogastric tube (NGT) is placed through the nose and brought out the mouth with either a finger placed inside the mouth or by grasping the NGT with endoscopic forceps. A piece of black polyurethane foam is trimmed to the appropriate size and then secured to the NGT with a size 2–0, or larger, suture on a straight needle. The NGT may require trimming to accommodate the length of the foam. It is important that all drain holes are covered by the foam to prevent suctioning up tissues into the drain hole as this would prevent further transmission of negative pressure to the foam.
The method we most commonly use to place the foam is the “Piggyback” method. In this method a suture is secured to the tip of the NGT and then a loop is created. The loop is then grasped with endoscopic forceps and the foam guided into place with the endoscope. Placing the NGT to suction prior to removing the endoscope will help prevent dislodgment of the foam once it is in place. Suction is provided by connecting the NGT to the Wound V.A.C.™ (Lifecell, Bridgewater, NJ) unit by using a short interconnecting piece of tubing with universal tubing connectors on both ends. The Wound V.A.C.™ pressure settings used in all patients were -175 mmHg, continuous, and high intensity.
E-Vac therapy is a cavity focused treatment method. The goal of therapy is to achieve complete closure of the cavity. Defect closure is the by-product of a successfully closed cavity. Adequate treatment of the cavity with irrigation, debridement, and placement of the foam within the cavity can result in immediate and significant reduction in the systemic inflammatory response, sepsis and septic shock. Inadequate treatment or failure to recognize an associated cavity severely limits the therapeutic potential of E-Vac therapy. Five of the six patients in this study presented with a sizeable cavity and were treated with foam placement within the cavity (extraluminal therapy). Patients with no discernable cavity (patient #1) and patients in whom only small cavities remained following extraluminal therapy (patient #2, 3, 4, 5, 6), were treated with placing the foam within the esophageal or gastric lumen adjacent to the defect (intraluminal therapy).
Foam changes
Continued exposure to large volumes of secretions and fluids causes gradual buildup of biologic material on and within the foam. Overtime this reduces the suctioning power of the system. Also, the longer the foam is in contact with the tissues, the greater the amount of tissue in-growth that occurs. Therefore, E-Vac therapy requires the foam to be changed every 3–7 days. The foam can be removed by first flushing saline through the NGT and then grasping the suture or the NGT with rat tooth forceps placed through the endoscope. The foam can also be removed by pulling on the NGT, while a finger is placed inside the mouth. When the foam is fairly fixed, the scope can be manipulated between the foam and the adjacent tissue.
It is important to perform foam changes on an on-demand basis during the acute infectious period. During this time period thick secretions and necrotic debris can rapidly degrade the effectiveness of the foam, decrease the level of suction, or cause an abrupt blockage in the system. In order to allow prompt recognition of the need to change out the foam we have now started drawing procalcitonin (PCT) levels as an early warning sign of the ineffectiveness of the foam or a blockage in the system. PCT levels are ideally drawn either before or immediately after starting E-Vac therapy, then daily until resolution of the systemic inflammatory response. Proper placement of E-Vac therapy should result in a significant decline in PCT levels each subsequent day until levels are within normal range. A foam change is needed if the PCT level increases or fails to decrease when above normal range.
Discontinuing therapy
We stop E-Vac therapy once the cavity is covered with granulation tissue, is 1 cm or less in depth, and appears to be sealed on endoscopic examination. We prefer CT with oral contrast to confirm complete closure after discontinuing E-Vac therapy. If closure of the leak or perforation is confirmed the patient is started on a liquid diet and advanced as tolerated to a soft or regular diet.
Other imaging considerations
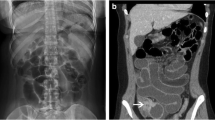
Performing a CT prior to the initial placement of E-Vac therapy can help to identify the presence of a cavity that will need to be treated or fluid collections that will not be amenable to drainage with E-Vac therapy alone. For this same reason a CT can be helpful in identifying reasons for a poor initial response to E-Vac therapy or an unexpected clinical deterioration during E-Vac therapy.
Study endpoints
Complete closure of the perforation or leak using E-Vac therapy was evaluated as the primary endpoint. Thirty-day mortality rate, total duration of E-Vac therapy, hospital length of stay (from the start of E-Vac therapy), and any complications directly related to E-Vac therapy were evaluated as secondary endpoints.
Results
E-Vac therapy treatment characteristics and results are given in Table 2. All (6/6) patients had complete closure of their perforation or leak after an average of 35.8 days (range 7–69 days) of E-Vac therapy and 7.2 different E-Vac changes (range 2–12 changes). Foam changes occurred on average every 4.8 days (range 2–9 days). The average time from the start of E-Vac therapy to complete closure of the perforation or leak was 40.2 days (range 7–69 days). In five patients E-Vac therapy was discontinued after complete closure was seen on endoscopy. In patient #2 E-Vac therapy was stopped when only a shallow depression could be seen extending from the base of the cavity. A follow-up endoscopy 2 weeks later showed complete closure of the cavity. Imaging (CT with oral contrast, esophagram, or upper GI) was used in all patients to confirm complete closure.
Following complete closure, five patients were started on a liquid diet and could tolerate a soft or a regular diet prior to leaving the hospital. Patient #5 could not be started on a diet as he remained on mechanical ventilation. Patient #4 was the only patient to have symptoms of dysphagia following E-Vac therapy. This patient had severe dysphagia from an esophagogastric anastomotic stricture prior to her iatrogenic perforations. Following E-Vac therapy her dysphagia had actually improved and was now able to tolerate a soft diet.
Additional radiologic drainage procedures were needed in patients #1, 2, and 3. Patient #5 had a thoracoscopic drainage procedure of a suspicious pleural fluid collection during E-Vac therapy. In all patients, E-Vac therapy was the primary method used to obtain complete closure. In two patients adjunctive endoscopic therapies were performed during E-Vac therapy. In patient #2 SurgiMend™ (TEI Biosciences, Boston, MA), an acellular matrix, was placed into the shallow depression at the base of the cavity. In patient #4 E-Vac therapy was stopped after 13 days when the cavity endoscopically appeared closed. E-Vac therapy was restarted the next day due to tachycardia and the appearance of a residual cavity on CT. Complete closure of the cavity was seen on endoscopy 2 weeks later. Two over the scope clips were placed to close the remaining mucosal defect. Complete closure of the cavity was confirmed on a follow-up esophagram. The patient was discharged home 4 days later on a regular diet.
Within 72 h after starting E-Vac therapy no patients required vasopressor support. Average stay in the intensive care unit after starting E-Vac therapy was 21.5 days (range 4–73 days). Hospital length of stay after starting E-Vac therapy was 42.7 days (21–73 days) (Table 3). There were no complications directly related to E-Vac therapy during a combined total of 215 E-Vac therapy days and 42 different E-Vac therapy changes.
No deaths occurred in the first 30 days following the start of E-Vac therapy or before complete closure of the perforation or leak (Table 3). Patient #5 had poor pulmonary function due to a history of smoking and a prior episode of acute respiratory distress syndrome (ARDS) that required tracheostomy tube placement. Upon presentation to the ED for his esophageal perforation he required immediate intubation and mechanical ventilation. Following complete closure of his perforation he was transferred to an LTAC facility for continued ventilatory assistance. The patient died 13 days later following an abbreviated laparotomy for an incarcerated inguinal hernia. An upper endoscopy was performed at the time and showed a completely closed perforation. The exact reason for his acute decompensation is not clear but certainly was attributable to worsening lung function and poor pulmonary reserve.
Discussion
In our study all perforations and leaks were successfully closed with E-Vac therapy and no deaths occurred within 30 days after starting E-Vac therapy. In our review of the literature we found seven major studies (case series with five or more patients) using E-Vac therapy to treat esophageal leaks and perforations [8, 10–15]. Closure rates were shown in six of the seven studies. The average closure rate of these six studies was 89.2 % (91/102 patients, range 84.4–100 %) [8, 10, 11, 13–15]. Mortality rates were reported in all seven studies and the average mortality rate was 10.1 % (12/119 patients, range 0–15.6 %) [8, 10–15].
The average duration of E-Vac therapy (35.8 days) in our study was longer than the average duration of therapy in the other E-Vac studies (range 11–28 days) [8, 10, 11, 13–15]. One contributing factor for this difference could be our foam change interval. Compared to the other E-Vac studies, our average changing interval was almost 2 days longer. We originally favored slightly longer intervals between changes to reduce the total number of endoscopies needed for each patient. Upon further evaluation it appears from our preliminary data (unpublished) that waiting longer than 4 days between each foam change increases the duration of E-Vac therapy. We have now switched to changing intervals of every 3–4 days and our continuing to evaluate this issue. Another contributing factor in our longer average treatment duration could be a delay in receiving E-Vac therapy. Two of the studies we reviewed showed a delay of >24 h before starting E-Vac therapy was associated with longer durations of E-Vac therapy [8, 15]. In our study all but one patient had a delay of >24 h before starting E-Vac therapy. The only patient without a delay in starting E-Vac therapy (patient #1) had the shortest duration of E-Vac therapy in our study (7 days). Unfortunately, delays in treatment will continue to occur when E-Vac therapy is used as a rescue therapy.
This is a small case series of six patients; therefore, it is difficult to compare the outcomes of this current study using E-Vac therapy with reported outcomes from large studies of endoscopic stent placement or surgical repair/resection. The patients in the current study were also either poor candidates or had already failed such methods. E-Vac therapy compares favorably (no 30-day mortality) to the mortality rates associated with stent placement (7.3–19 %) and surgical repair (9.5–13.1 %) for esophageal perforations [4, 16]. E-Vac therapy averaged 5 weeks (35.8 days) compared to 6–8 weeks for stent placement and a recovery period of almost 5 weeks (33 days) with surgical therapy [4, 7]. There were no complications directly related to the use of E-Vac therapy. Stent placement has a combined morbidity of 34 %, most often from stent migration. Endoscopic reintervention and surgical intervention were required in 25 and 13 % of patients, respectively [17]. Tracheostomy tube placement, post-operative infection, and a recurrent leak followed operative repair in 22, 53, and 50 % of patients, respectively [18, 19].
Our initial results with the use of E-Vac therapy are very encouraging. To our knowledge there have been no prior reports on the use of E-Vac therapy here in the USA. This study, however, is limited due to the small number of patients. Some obvious limitations also exist with the use of E-Vac therapy. Since no FDA approved device currently exists, E-Vac therapy requires adaptation and off-label use of the current Wound V.A.C.™ (Lifecell, Bridgewater, NJ) device. E-Vac therapy also requires multiple endoscopic procedures and proper placement of the foam can be technically demanding at times.
Conclusion
E-Vac therapy is a promising treatment method for upper gastrointestinal leaks and perforations. In our study E-Vac therapy allowed complete closure of all perforations and leaks even in patients that had failed other therapies. A larger group of less heterogeneous patients will be needed to further evaluate the appropriate changing interval for E-Vac therapy. Future prospective controlled studies will be needed to validate our current results and to evaluate the use of E-Vac therapy as a possible primary treatment modality.
References
Dunnington GL, DeMeester TR (1993) Outcome effect of adherence to operative principles of Nissen fundoplication by multiple surgeons. The Department of Veterans Affairs Gastroesophageal Reflux Disease Study Group. Am J Surg 166(6):654–657
Schauer PR, Meyers WC, Eubanks S, Norem RF, Franklin M, Pappas TN (1996) Mechanisms of gastric and esophageal perforations during laparoscopic Nissen fundoplication. Ann Surg 223(1):43–52
Zhang LP, Chang R, Matthews BD, Awad M, Meyers B, Eagon JC, Brunt LM (2014) Incidence, mechanisms, and outcomes of esophageal and gastric perforation during laparoscopic foregut surgery: a retrospective review of 1,223 foregut cases. Surg Endosc 28(1):85–90. doi:10.1007/s00464-013-3167-1
Biancari F, D’Andrea V, Paone R, Marco C, Savino G, Koivukangas V, Saarnio J, Lucenteforte E (2013) Current treatment and outcome of esophageal perforations in adults: systematic review and meta-analysis of 75 studies. World J Surg 37(5):1051–1059. doi:10.1007/s00268-013-1951-7
Hasimoto CN, Cataneo C, Eldib R, Thomazi R, Pereira RSdC, Minossi JG, Cataneo AJM (2013) Efficacy of surgical versus conservative treatment in esophageal perforation: a systematic review of case series studies. Acta Cirúrgica Brasileira/Sociedade Brasileira Para Desenvolvimento Pesquisa Em Cirurgia 28(4):266–271
Persson S, Elbe P, Rouvelas I, Lindblad M, Kumagai K, Lundell L, Nilsson M, Tsai JA (2014) Predictors for failure of stent treatment for benign esophageal perforations—a single center 10-year experience. World J Gastroenterol 20(30):10613–10619. doi:10.3748/wjg.v20.i30.10613
Dasari BVM, Neely D, Kennedy A, Spence G, Rice P, Mackle E, Epanomeritakis E (2014) The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg 259(5):852–860. doi:10.1097/SLA.0000000000000564
Weidenhagen R, Hartl WH, Gruetzner KU, Eichhorn ME, Spelsberg F, Jauch KW (2010) Anastomotic leakage after esophageal resection: new treatment options by endoluminal vacuum therapy. Ann Thorac Surg 90(5):1674–1681. doi:10.1016/j.athoracsur.2010.07.007
Wedemeyer J, Brangewitz M, Kubicka S, Jackobs S, Winkler M, Neipp M, Klempnauer J, Manns MP, Schneider AS (2010) Management of major postsurgical gastroesophageal intrathoracic leaks with an endoscopic vacuum-assisted closure system. Gastrointest Endosc 71(2):382–386. doi:10.1016/j.gie.2009.07.011
Ahrens M (2010) Drainage of esophageal leakage using endoscopic vacuum therapy: a prospective pilot study. Endoscopy 42(9):693–698
Brangewitz M, Voigtländer T, Helfritz FA, Lankisch TO, Winkler M, Klempnauer J, Manns MP, Schneider AS, Wedemeyer J (2013) Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy 45(6):433–438. doi:10.1055/s-0032-1326435
Schniewind B, Schafmayer C, Voehrs G, Egberts J, von Schoenfels W, Rose T, Kurdow R, Arlt A, Ellrichmann M, Jürgensen C, Schreiber S, Becker T, Hampe J (2013) Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc 27(10):3883–3890. doi:10.1007/s00464-013-2998-0
Schorsch T, Müller C, Loske G (2014) Endoskopische Vakuumtherapie von Perforationen und Anastomoseninsuffizienzen des Ösophagus (German). Endoscopic vacuum therapy of perforations and anastomotic insufficiency of the esophagus (English). 85 (12):1081–1093. doi:10.1007/s00104-014-2764-4
Bludau M, Hölscher AH, Herbold T, Leers JM, Gutschow C, Fuchs H, Schröder W (2014) Management of upper intestinal leaks using an endoscopic vacuum-assisted closure system (E-VAC). Surg Endosc 28(3):896–901. doi:10.1007/s00464-013-3244-5
Heits N, Stapel L, Reichert B, Schafmayer C, Schniewind B, Becker T, Hampe J, Egberts J-H (2014) Endoscopic endoluminal vacuum therapy in esophageal perforation. Ann Thorac Surg 97(3):1029–1035. doi:10.1016/j.athoracsur.2013.11.014
Biancari Fausto, Saarnio Juha, Mennander Ari, Hypén Linda, Salminen Paulina, Kuttila Kari, Victorzon Mikael, Böckelman Camilla, Tarantino Enrico, Tiffet Olivier, Koivukangas Vesa, Søreide Jon Arne, Viste Asgaut, Bonavina Luigi, Vidarsdóttir Halla, Gudbjartsson Tomas (2014) Outcome of patients with esophageal perforations: a multicenter study. World J Surg 38(4):902–909. doi:10.1007/s00268-013-2312-2
van Boeckel PGA, Sijbring A, Vleggaar FP, Siersema PD (2011) Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther 33(12):1292–1301. doi:10.1111/j.1365-2036.2011.04663.x
Lindenmann Joerg, Matzi Veronika, Neuboeck Nicole, Anegg Udo, Maier Alfred, Smolle Josef, Smolle-Juettner Freyja (2013) Management of esophageal perforation in 120 consecutive patients: clinical impact of a structured treatment algorithm. J Gastrointest Surg 17(6):1036–1043. doi:10.1007/s11605-012-2070-8
Sulpice L, Rayar M, Laviolle B, Cunin D, Merdrignac A, Boudjema K, Meunier Bernard (2013) Surgical treatment of esophageal perforations: the importance of a primary repair. Surg Today 43(7):727–731. doi:10.1007/s00595-012-0328-0
Acknowledgments
The authors would like to thank Drs. Edson Cheung, Robert Hebeler, Baron Hamman, Albert Henry, Bruce Smith, Howard Derrick, and the entire Trauma/Critical Care Staff (Drs. Michael Foreman, Matthew Lovitt, Laura Petrey, James Carroll, Edward Taylor, and Geoffrey Funk) for their expertise in helping manage these patients; YehShen McShan, BA, Arlen Waclawczyk, BS, and Tammy Fisher, RN, MBA, ACHE for their research related support; and finally, the Seeger Surgical Fund of the Baylor Healthcare System Foundation for financial support of this research study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Nathan Smallwood, Steven Leeds, James W. Fleshman, and J. S. Burdick have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (WMV 10104 kb)
Supplementary material 2 (WMV 19760 kb)
Supplementary material 3 (WMV 77143 kb)
Supplementary material 4 (WMV 66518 kb)
Rights and permissions
About this article
Cite this article
Smallwood, N.R., Fleshman, J.W., Leeds, S.G. et al. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc 30, 2473–2480 (2016). https://doi.org/10.1007/s00464-015-4501-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4501-6