Abstract
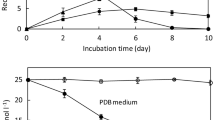
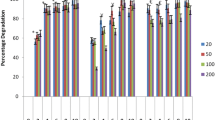
The use of biomaterials or microorganisms in PAHs degradation had presented an eye-catching performance. Pleurotus eryngii is a white rot fungus, which is easily isolated from the decayed woods in the tropical rain forest, used to determine the capability to utilize naphthalene, a two-ring polycyclic aromatic hydrocarbon as source of carbon and energy. In the meantime, biotransformation of naphthalene to intermediates and other by-products during degradation was investigated in this study. Pleurotus eryngii had been incubated in liquid medium formulated with naphthalene for 14 days. The presence of metabolites of naphthalene suggests that Pleurotus eryngii begin the ring cleavage by dioxygenation on C1 and C4 position to give 1,4-naphthaquinone. 1,4-Naphthaquinone was further degraded to benzoic acid, where the proposed terepthalic acid is absent in the cultured extract. Further degradation of benzoic acid by Pleurotus eryngii shows the existence of catechol as a result of the combination of decarboxylation and hydroxylation process. Unfortunately, phthalic acid was not detected in this study. Several enzymes, including manganese peroxidase, lignin peroxidase, laccase, 1,2-dioxygenase and 2,3-dioxygenase are enzymes responsible for naphthalene degradation. Reduction of naphthalene and the presence of metabolites in liquid medium showed the ability of Pleurotus eryngii to utilize naphthalene as carbon source instead of a limited glucose amount.





Similar content being viewed by others
References
Pozdnyakova NN, Rodakiewicz-Nowak J, V. Turkovskaya O, Haber J (2006) Oxidative degradation of polyaromatic hydrocarbons and their derivatives catalyzed directly by the yellow laccase from Pleurotus ostreatus D1. J Mol Catal Enzym 41:8–15
Zhang XX, Cheng SP, Zhu CJ, Sun SL (2006) Microbial PAH-degradation in soil: degradation pathways and contributing factors. Pedosphere 16:555–565
Kakareka SV (2002) Sources of persistent organic pollutants emission on the territory of Belarus. Atmos Environ 36:1407–1419
Jones KC, de Voogt P (1999) Persistent organic pollutants (POPs): state of the science. Environ Pollut 100:209–221
Gon HDVD, Bolscher MVH, Visschedijk A, Zandveld P (2007) Emissions of persistent organic pollutants and eight candidate POPs from UNECE-Europe in 2000, 2010 and 2020 and the emission reduction resulting from implementation of the UNECE POP protocol. Atmos Environ 41:9245–9261
Lohmann R, Breivik K, Dachs J, Muir D (2007) Global fate of POPs: current and future research directions. Environ Pollut 150:150–165
Hadibarata T, Yusoff ARM, Aris A, Kristanti RA (2012) Identification of naphthalene metabolism by white rot fungus Armillaria sp. F022. J Environ Sci 24:728–732
Rivas FJ (2006) Polycyclic aromatic hydrocarbons sorbed on soils: a short review of chemical oxidation based treatments. J Hazard Mater 138:234–251
Semple KT, Doick KJ, Wick LY, Harms H (2007) Microbial interactions with organic contaminants in soil: definitions, processes and measurement. Environ Pollut 150:166–176
Pathak H, Kantharia D, Malpani A, Madamwar D (2009) Naphthalene degradation by Pseudomonas sp. HOB1: in vitro studies and assessment of naphthalene degradation efficiency in simulated microcosms. J Hazard Mater 166:1466–1473
Mrozik A, Labuzek S, Piotrowska-Seget Z (2005) Changes in fatty acid composition in Pseudomonas putida and Pseudomonas stutzeri during naphthalene degradation. Microbiol Res 160:149–157
Dou J, Liu X, Ding A (2009) Anaerobic degradation of naphthalene by the mixed bacteria under nitrate reducing conditions. J Hazard Mater 165:325–331
Lin C, Gan L, Chen ZL (2010) Biodegradation of naphthalene by strain Bacillus fusiformis (BFN). J Hazard Mater 182:771–777
Rockne KJ, Strand SE (2001) Anaerobic biodegradation of naphthalene, phenanthrene, and biphenyl by a denitrifying enrichment culture. Water Res 35:291–299
Hadibarata T, Yusoff ARM, Aris A, Salmiati, Hidayat T, Kristanti RA (2011) Decoloration of azo, triphenylmethane and anthraquinone dyes by laccase of a newly isolated Armillaria sp. F022. Water Air Soil Pollut 223:1045–1054
Gao L, Liu X (2010) Effects of carbon concentrations and carbon to nitrogen ratios on sporulation of two biological control fungi as determined by different culture methods. Mycopathologia 169:475–481
Rajderkar N (1966) Certain chemical requirements for growth and sporulation of alternaria species. Mycopathologia 30:172–176
Lee CL, Hung HK, Wang JJ, Pan TM (2007) Improving the ratio of Monacolin K to Citrinin production of Manascus purpureus NTU 568 under dioscorea medium through the mediation of pH value and ethanol addition. J Agric Food Chem 55:6493–6502
Hadibarata T, Khudhair AB, Salim MR (2012) Breakdown products in the metabolic pathway of anthracene degradation by a ligninolytic fungus Polyporus sp. S133. Water Air Soil Pollut 223:2201–2208
Hadibarata T, Kristanti RA (2012) Identification of metabolites from benzo[a]pyrene oxidation by ligninolytic enzymes of Polyporus sp. S133. J Environ Manag 111:115–119
Zharare GE, Kabanda SM, Poku JZ (2010) Effects of temperature and hydrogen peroxide on mycelial growth of eight Pleurotus strains. Sci Hortic 125:95–102
Hadibarata T, Kristanti RA (2012) Fate and cometabolic degradation of benzo[a]pyrene by white-rot fungus Armillaria sp. F022. Bioresour Technol 107:314–318
Zeinali M, Vossoughi M, Ardestani SK (2008) Naphthalene metabolism in Nocardia otitidiscaviarum strain TSH1, a moderately thermophilic microorganism. Chemosphere 72:905–909
Acknowledgments
A part of this project was financially supported by Universiti Teknologi Malaysia (RUG Vote QJ1.3000.2522.02H65) and Ministry of High Education, Malaysia (ERGS Vote R.J130000.7822.4L053).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hadibarata, T., Teh, Z.C., Rubiyatno et al. Identification of naphthalene metabolism by white rot fungus Pleurotus eryngii . Bioprocess Biosyst Eng 36, 1455–1461 (2013). https://doi.org/10.1007/s00449-013-0884-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-0884-8