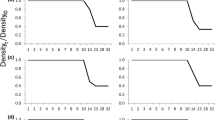
Abstract
Conditions experienced in early developmental stages can have long-term consequences for individual fitness. High intraspecific density during the natal period can affect juvenile and eventually adult growth rates, metabolism, immune function, survival, and fecundity. Despite the important ecological and evolutionary effects of early developmental density, the form of the relationship between natal density and resulting juvenile phenotype is poorly understood. To test competing hypotheses explaining responses to intraspecific density, we experimentally manipulated the initial larval density of ringed salamanders (Ambystoma annulatum), a pond-breeding amphibian, over 11 densities. We modeled the functional form of the relationship between natal density and juvenile traits, and compared the relative support for the various hypotheses based on their goodness of fit. These functional form models were then used to parameterize a simple simulation model of population growth. Our data support non-additive density dependence and presents an alternate hypothesis to additive density dependence, self-thinning and Allee effects in larval amphibians. We posit that ringed salamander larvae may be under selective pressure for tolerance to high density and increased efficiency in resource utilization. Additionally, we demonstrate that models of population dynamics are sensitive to assumptions of the functional form of density dependence.



Similar content being viewed by others
References
Anderson TL, Semlitsch RD (2014) High intraguild predator density induces thinning effects on and increases temporal overlap with prey populations. Popul Ecol 56:265–273. doi:10.1007/s10144-013-0419-9
Bates DM, Watts DG (1988) Nonlinear regression analysis and its applications. Wiley, New York
Bellows TS (1981) The descriptive properties of some models for density dependence. J Anim Ecol 50:139. doi:10.2307/4037
Berner D (2011) Size correction in biology: how reliable are approaches based on (common) principal component analysis? Oecologia 166:961–971. doi:10.1007/s00442-011-1934-z
Berven KA, Gill DE (1983) Interpreting geographic variation in life-history traits. Am Zool 23:85–97
Biek R, Funk WC, Maxell BA, Mills LS (2002) What is missing in amphibian decline research: insights from ecological sensitivity analysis. Conserv Biol 16:728–734. doi:10.1046/j.1523-1739.2002.00433.x
Brook BW, Bradshaw CJA (2006) Strength of evidence for density dependence in abundance time series of 1198 species. Ecology 87:1445–1451
Bult TP, Riley SC, Haedrich RL et al (1999) Density-dependent habitat selection by juvenile Atlantic salmon (Salmo salar) in experimental riverine habitats. Can J Fish Aquat Sci 56:1298–1306. doi:10.1139/f99-074
Chelgren ND, Rosenberg DK, Heppell SS, Gitelman AI (2006) Carryover aquatic effects on survival of metamorphic frogs during pond emigration. Ecol Appl 16:250–261
Cole LC (1954) The population consequences of life history phenomena. Q Rev Biol 29:103–137
Cosentino BJ, Schooley RL, Phillips CA (2011) Spatial connectivity moderates the effect of predatory fish on salamander metapopulation dynamics. Ecosphere 2:art95. doi:10.1890/ES11-00111.1
Crawley MJ (2013) The R book, 2nd edn. Wiley, West Sussex
Crespi EJ, Denver RJ (2004) Ontogeny of corticotropin-releasing factor effects on locomotion and foraging in the Western spadefoot toad (Spea hammondii). Horm Behav 46:399–410. doi:10.1016/j.yhbeh.2004.03.011
Davis AK (2012) Investigating the optimal rearing strategy for Ambystoma salamanders using a hematological stress index. Herpetol Conserv Biol 7:95–100
Davis AK, Maerz JC (2009) Effects of larval density on hematological stress indices in salamanders. J Exp Zool 311A:697–704. doi:10.1002/jez.557
De Kogel CH (1997) Long-term effects of brood size manipulation on and sex-specific mortality of morphological development offspring. J Anim Ecol 66:167–178
Earl JE, Luhring TM, Williams BK, Semlitsch RD (2011) Biomass export of salamanders and anurans from ponds is affected differentially by changes in canopy cover. Freshw Biol 56:2473–2482. doi:10.1111/j.1365-2427.2011.02672.x
Einum S, Sundt-Hansen L, Nislow KH (2006) The partitioning of density-dependent dispersal, growth and survival throughout ontogeny in a highly fecund organism. Oikos 113:489–496. doi:10.1111/j.2006.0030-1299.14806.x
Figiel CR, Semlitsch RD (1990) Population variation in survival and metamorphosis of larval salamanders (Ambystoma maculatum) in the presence and absence of fish predation. Copeia 1990:818–826
Gill DE (1978) On selection at high population density. Ecology 59:1289–1291
Glennemeier KA, Denver RJ (2002) Role for corticoids in mediating the response of Rana pipiens tadpoles to intraspecific competition. J Exp Zool 292:32–40. doi:10.1002/jez.1140
Harper EB, Semlitsch RD (2007) Density dependence in the terrestrial life history stage of two anurans. Oecologia 153:879–889. doi:10.1007/s00442-007-0796-x
Herrando-Perez S, Delean S, Brook BW, Bradshaw CJA (2012) Density dependence: an ecological Tower of Babel. Oecologia 170:585–603. doi:10.1007/s00442-012-2347-3
Hocking DJ, Rittenhouse TAG, Rothermel BB et al (2008) Breeding and recruitment phenology of amphibians in Missouri oak-hickory forests. Am Midl Nat 160:41–60. doi:10.1674/0003-0031(2008)160
Lindström J (1999) Early development and fitness in birds and mammals. Trends Ecol Evol 14:343–348
Loman J (2004) Density regulation in tadpoles of Rana temporaria: a full pond field experiment. Ecology 85:1611–1618. doi:10.1890/03-0179
May RM, Oster GF (1976) Bifurcations and dynamic complexity in simple ecological models. Am Nat 110:573–599
Monaghan P (2008) Early growth conditions, phenotypic development and environmental change. Philos Trans Biol Sci 363:1635–1645. doi:10.1098/rstb.2007.0011
Morin PJ (1988) Functional redundancy, non-additive interactions, and supply-side dynamics in experimental pond communities. Ecology 76:133–149
Mott CL, Maret TJ (2011) Species-specific patterns of agonistic behavior among larvae of three syntopic species of Ambystomatid salamanders. Copeia 2011:9–17. doi:10.1643/CE-09-065
Nyman S, Wilkinson RF, Hutcherson JE (1993) Cannibalism and size relations in a cohort of larval ringed salamanders (Ambystoma annulatum). J Herpetol 27:78–84
Ousterhout BH, Luhring TM, Semlitsch RD (2014) No evidence of natal habitat preference induction in juveniles with complex life histories. Anim Behav 93:237–242. doi:10.1016/j.anbehav.2014.04.035
Ousterhout BH, Anderson TL, Drake DL et al (2015) Habitat traits and species interactions differentially affect abundance and body size in pond-breeding amphibians. J Anim Ecol 84:914–924
Pechmann JHK (1995) Use of large field enclosures to study the terrestrial ecology of pond-breeding amphibians. Herpetologica 51:434–450
Peterman WE, Locke JL, Semlitsch RD (2013) Spatial and temporal patterns of water loss in heterogeneous landscapes: using plaster models as amphibian analogues. Can J Zool 140:135–140
Petranka JW (1998) Salamanders of the United States and Canada. Smithsonian Institution Press, Wasington, DC
Post JR, Parkinson EA, Johnston NT (1999) Density-dependent processes in strucutred fish populations: interaction strengths in whole-lake experiments. Ecol Monogr 69:155–175
R Core Team (2015) R: A language and environment for statistical computing
Richardson JL (2012) Divergent landscape effects on population connectivity in two co-occurring amphibian species. Mol Ecol 21:4437–4451. doi:10.1111/j.1365-294X.2012.05708.x
Rollinson N, Hutchings JA (2013) The relationship between offspring size and fitness: integrating theory and empiricism. Ecology 94:315–324
Rot-Nikcevic I, Denver RJ, Wassersug RJ (2005) The influence of visual and tactile stimulation on growth and metamorphosis in anuran larvae. Funct Ecol 19:1008–1016. doi:10.1111/j.1365-2435.2005.01051.x
Scott DE (1990) Effects of larval density in Ambystoma opacum: an experiment in large-scale field enclosures. Ecology 71:296–306
Scott DE (1994) The effect of larval density on adult demographic traits in Ambystoma opacum. Ecology 75:1383–1396
Semlitsch RD (1987) Density-dependent growth and fecundity in the paedomorphic salamander Ambystoma talpoideum. Ecology 68:1003–1008
Semlitsch RD, Caldwell JP (1982) Effects of density on growth, metamorphosis, and survivorship in tadpoles of Scaphiopus holbrooki. Ecology 63:905–911
Semlitsch RD, Scott DE, Pechmann JHK (1988) Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology 69:184–192
Smith DC (1987) Adult recruitment in chorus frogs: effects of size and date at metamorphosis. Ecology 68:344–350
Smith-Gill SJ, Gill DE (1978) Curvilinearities in the competition equations: an experiment with ranid tadpoles. Am Nat 112:557–570
Van Buskirk J (2005) Local and landscape influence on amphibian occurance and abundance. Ecology 86:1936–1947
Van Buskirk J, Smith DC (1991) Density-dependent population regulation in a salamander. Ecology 72:1747–1756
Vonesh JR (2005) Sequential predator effects across three life stages of the African tree frog, Hyperolius spinigularis. Oecologia 143:280–290. doi:10.1007/s00442-004-1806-x
Walls SC, Jaeger RG (1987) Aggression and exploitation as mechanisms of competition in larval salamanders. Can J Zool 65:2938–2944
Wilbur HM (1976) Density-dependent aspects of metamorphosis in Ambystoma and Rana sylvatica. Ecology 57:1289–1296
Wilbur HM (1977) Density-dependent aspects of growth and metamorphosis in Bufo americanus. Ecology 58:196–200
Wilbur HM (1980) Complex life cycles. Annu Rev Ecol Evol Syst 11:67–93
Wright SJ (2002) Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130:1–14. doi:10.1007/s004420100809
Acknowledgments
We thank T. Anderson, D. Drake, C. Farmer, A. Milo, and K. Proffit for help collecting data. T. Anderson, R. Holdo, D. Kesler, and H. Wilbur provided comments on earlier drafts of this manuscript. We also thank R. Holdo for his assistance with the population model. This work was supported by a National Science Foundation Graduate Research Fellowship to B. H. Ousterhout and the Department of Defense (SERDP RC-2155). Animals were collected and maintained under Missouri Department of Conservation permit 14922 and ACUC Protocol 7403. All experiments comply with the current laws of the United States of America. The authors declare no conflict of interest.
Author contribution statement
BHO and RDS conceived and designed the experiments. BHO performed the experiments, analyzed the data, and wrote the manuscript. RDS provided editorial advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Steven Kohler.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ousterhout, B.H., Semlitsch, R.D. Non-additive response of larval ringed salamanders to intraspecific density. Oecologia 180, 1137–1145 (2016). https://doi.org/10.1007/s00442-015-3516-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3516-y