Abstract
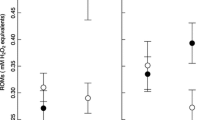
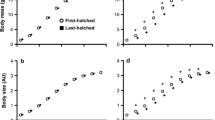
The short favorable period of time available for the growth in seasonal environments could constrain the resources allocation between growth and other life-history traits, and the short-term fitness benefits of increased growth rate may prevail over other functions. Accelerated growth rates have been associated with long-term deleterious consequences (e.g., decreased lifespan), and recently oxidative stress (the imbalance between pro-oxidants generation and antioxidant defenses) has been suggested as a mediator of these effects. Here, we examined the impact of elevation on growth rate and self-maintenance parameters (resting metabolism, oxidative damage, and antioxidant defenses) of coal tit chicks (Periparus ater). We predicted that the shorter favorable season at the higher-elevation site could lead to a reallocation of resources towards growth at the expense of self-maintenance processes. We found that chicks at high elevation grew significantly faster in terms of body mass and body size. Chicks from the high-elevation site presented higher resting metabolism, higher oxidative damage level, but similar antioxidant defenses, compared to low-elevation chicks. Interestingly, the chicks exhibiting the better antioxidant defenses at 7 days were also those with the highest resting metabolic rate, and the chicks that grew at the faster rate within the high-elevation site were those with the highest levels of oxidative damage on DNA. Our study supports the idea that increasing elevation leads to a higher growth rate in coal tit chicks, possibly in response to a shorter favorable season. In accordance with life-history theory, a bigger investment in growth was done at the expense of body maintenance, at least in terms of oxidative stress.


Similar content being viewed by others
References
Abrams P, Leimar O, Nylin S, Wiklund C (1996) The effect of flexible growth rates on optimal sizes and development times in a seasonal environment. Am Nat 147:381–395
Alonso-Alvarez C, Bertrand S, Faivre B, Sorci G (2007) Increased susceptibility to oxidative damage as a cost of accelerated somatic growth in zebra finches. Funct Ecol 21:873–879
Arendt JD (1997) Adaptive intrinsic growth rates: an integration across taxa. Q Rev Biol 72:149–177
Badyaev AV, Ghalambor CK (2001) Evolution of life histories along elevational gradients: trade-off between parental care and fecundity. Ecology 82:2948–2960
Barja G (2007) Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuvenation Res 10:215–223
Beckman K, Ames B (1998) The free radical theory of aging matures. Physiol Rev 78:547–581
Betts MM (1955) The food of titmice in oak woodland. J Anim Ecol 24:282–323
Bize P, Metcalfe NB, Roulin A (2006) Catch-up growth strategies differ between body structures: interactions between age- and structure-specific growth in wild nestling Alpine Swifts. Funct Ecol 20:857–864
Bize P, Devevey G, Monaghan P et al (2008) Fecundity and survival in relation to resistance to oxidative stress in a free-living bird. Ecology 89:2584–2593
Bize P, Criscuolo F, Metcalfe N et al (2009) Telomere dynamics rather than age predict life expectancy in the wild. Proc R Soc B 276:1679–1683
Calow P (1982) Homeostasis and fitness. Am Nat 120:416–419
Careau V, Bergeron P, Garant D et al (2013) The energetic and survival costs of growth in free-ranging chipmunks. Oecologia 171:11–23
Conway C, Martin T (2000) Effects of ambient temperature on avian incubation behavior. Behav Ecol 11:178–188
Costantini D (2008) Oxidative stress in ecology and evolution: lessons from avian studies. Ecol Lett 11:1238–1251
Criscuolo F, Monaghan P, Nasir L, Metcalfe NB (2008) Early nutrition and phenotypic development: “catch-up” growth leads to elevated metabolic rate in adulthood. Proc R Soc B 275:1565–1570
Criscuolo F, Monaghan P, Proust A et al (2011) Costs of compensation: effect of early life conditions and reproduction on flight performance in zebra finches. Oecologia 167:315–323
Dittmar C, Elling W (2005) Phenological phases of common beech (Fagus sylvatica L.) and their dependence on region and altitude in southern Germany. Eur J For Res 125:181–188
Dmitriew CM (2011) The evolution of growth trajectories: what limits growth rate? Biol Rev 86:97–116
Dowling D, Simmons L (2009) Reactive oxygen species as universal constraints in life-history evolution. Proc R Soc B 276:1737–1745
Finkel T, Holbrook N (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Fletcher QE, Selman C, Boutin S et al (2013) Oxidative damage increases with reproductive energy expenditure and is reduced by food-supplementation. Evolution 67:1527–1536
Freeman S, Jackson WM (1990) Univariate metrics are not adequate to measure avian body size. Auk 107:69–74
Geiger S, Kaufffmann M, Le Maho Y et al (2012a) Of the importance of metabolic phases in the understanding of oxidative stress in prolonged fasting and refeeding. Physiol Biochem Zool 85:415–420
Geiger S, Le Vaillant M, Lebard T et al (2012b) Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol Ecol 21:1500–1510
Gotthard K (2008) Adaptive growth decisions in butterflies. Bioscience 58:222–230
Griffiths R, Double MC, Orr K, Dawson R (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Halliwell B, Gutteridge J (2007) Free radicals in biology and medicine. Oxford University Press, Oxford
Hayes JP (1989) Altitudinal and seasonal effects on aerobic metabolism of deer mice. J Comp Physiol B 159:453–459
Heidinger BJ, Blount JD, Boner W et al (2012) Telomere length in early life predicts lifespan. PNAS 109:1743–1748
Jefferson J, Simon J, Escudero E et al (2004) Increased oxidative stress following acute and chronic high-altitude exposure. High Alt Med Biol 5:61–69
Kim S-Y, Noguera JC, Morales J, Velando A (2011) Quantitative genetic evidence for trade-off between growth and resistance to oxidative stress in a wild bird. Evol Ecol 25:461–472
Lack D (1968) Ecological adaptations for breeding in birds. Methuen, London
Lee W-S, Monaghan P, Metcalfe NB (2013) Experimental demonstration of the growth rate–lifespan trade-off. Proc R Soc Lond B Biol Sci 280:20122370
Mangel M, Munch S (2005) A life-history perspective on short-and long-term consequences of compensatory growth. Am Nat 166:E155–E176
McVicar TR, Körner C (2012) On the use of elevation, altitude, and height in the ecological and climatological literature. Oecologia 171:335–337
Metcalfe N, Alonso Alvarez C (2010) Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct Ecol 24:984–996
Metcalfe N, Monaghan P (2001) Compensation for a bad start: grow now, pay later? TREE 16:254–260
Monaghan P, Metcalfe NB, Torres R (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12:75–92
Monrós JS, Belda EJ, Barba E (1998) Delays of the hatching dates in great tits Parus major: effects on breeding performance. Ardea 86:213–220
Naef Daenzer B, Keller LF (1999) The foraging performance of great and blue tits (Parus major and P. caeruleus) in relation to caterpillar development, and its consequences for nestling growth and fledging weight. J Anim Ecol 68:708–718
Naef-Daenzer B, Widmer F, Nuber M (2001) Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J Anim Ecol 70:730–738
Naef-Daenzer B, Nager RG, Keller L, Naef-Daenzer B (2004) Are hatching delays a cost or a benefit for great tit (Parus major) parents? Ardea 92:229–238
Naef-Daenzer B, Luterbacher J, Nuber M et al (2012) Cascading climate effects and related ecological consequences during past centuries. Clim Past 8:1527–1540
Noguera JC, Kim S-Y, Velando A (2012) Pre-fledgling oxidative damage predicts recruitment in a long-lived bird. Biol Lett 8:61–63
Nussey DH, Pemberton JM, Pilkington JG, Blount JD (2009) Life history correlates of oxidative damage in a free-living mammal population. Funct Ecol 23:809–817
Pellerin M, Delestrade A, Mathieu G et al (2012) Spring tree phenology in the Alps: effects of air temperature, altitude and local topography. Eur J For Res 131:1957–1965
Quinlivan EP, Gregory JF III (2008) DNA digestion to deoxyribonucleoside: a simplified one-step procedure. Anal Biochem 373:383–385
Reeve, Fowler, Partridge (2000) Increased body size confers greater fitness at lower experimental temperature in male Drosophila melanogaster. J Evol Biol 13:836–844
Richner H (1989) Habitat-specific growth and fitness in carrion crows (Corvus corone corone). J Anim Ecol 58:427–440
Ricklefs RE (1979) Patterns of growth in birds. V. A comparative study of development in the starling, common tern, and Japanese quail. Auk 96:10–30
Roff D (1980) Optimizing development time in a seasonal environment: the “ups and downs” of clinal variation. Oecologia 45:202–208
Rosa CE, Figueiredo MA, Lanes CFC et al (2008) Metabolic rate and reactive oxygen species production in different genotypes of GH-transgenic zebrafish. Comp Biochem Phys B 149:209–214
Salin K, Luquet E, Rey B et al (2012) Alteration of mitochondrial efficiency affects oxidative balance, development and growth in frog (Rana temporaria) tadpoles. J Exp Biol 215:863–869
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stier A, Reichert S, Massemin S et al (2012) Constraint and cost of oxidative stress on reproduction: correlative evidence in laboratory mice and review of the literature. Front Zool 9:37
Stier A, Bize P, Habold H et al (2014a) Mitochondrial uncoupling prevents cold-induced oxidative stress: a case study using UCP1 knock-out mice. J Exp Biol 217:624–630
Stier A, Viblanc VA, Massemin-Challet S et al (2014b) Starting with a handicap: phenotypic differences between early- and late-born king penguin chicks and their survival correlates. Funct Ecol. doi:10.1111/1365-2435.12204
Tarry-Adkins JL, Martin-Gronert MS, Chen JH et al (2008) Maternal diet influences DNA damage, aortic telomere length, oxidative stress, and antioxidant defense capacity in rats. FASEB J 22:2037–2044
Tarry-Adkins JL, Chen JH, Smith NS et al (2009) Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J 23:1521–1528
Tsuchiya Y, Takami Y, Okuzaki Y, Sota T (2012) Genetic differences and phenotypic plasticity in body size between high- and low-altitude populations of the ground beetle Carabus tosanus. J Evol Biol 25:1835–1842
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
Weathers W, Davidson C, Olson C et al (2002) Altitudinal variation in parental energy expenditure by white-crowned sparrows. J Exp Biol 205:2915–2924
Wood SN (2006) Generalized additive models: an introduction with R. CRC press, Miami
Zera A, Harshman L (2001) The physiology of life history trade-offs in animals. Annu Rev Ecol Sys 32:95–126
Zhong YC, Luo P, Jin G et al (2011) The dynamic changing profiles of peripheral white blood cell telomere length in populations of different ages living at different altitude areas. Zhonghua Yu Fang Yi Xue Za Zhi 42:502–505
Acknowledgments
We are grateful to G. Chagneau and O. Scholly for help with fieldwork, to Antoine Duparc for assistance with the statistical analysis of temperature data, and to the CNRS, The University of Strasbourg, and The CREA for funding. We are especially grateful to two anonymous reviewers and the handling editor for providing interesting and constructive comments on a previous draft of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Oliver P. Love.
F. Criscuolo and S. Massemin-Challet contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stier, A., Delestrade, A., Zahn, S. et al. Elevation impacts the balance between growth and oxidative stress in coal tits. Oecologia 175, 791–800 (2014). https://doi.org/10.1007/s00442-014-2946-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-2946-2