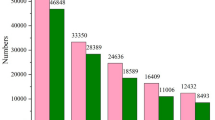
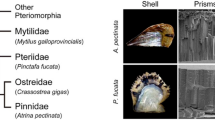
Abstract
Cristaria plicata, a bivalve widespread in Eastern Asia fresh water, is utilized as the freshwater pearl mussel in China. With a high economic value in pearl production, it is also an ideal object used for the studies on biomineralization in freshwater. In the research, we performed a large-scale sequencing of Cristaria plicata mantle transcriptome using Illumina HiSeq™ 2500, obtaining 98,501 unigenes with 67,817,724 bases. 22.28 and 16.64% of the unigenes were annotated in the NR and Swiss-Prot databases, respectively. Most of the annotated unigenes were homologous with proteins of Crassostrea gigas (47.4%) and some were similar to proteins of Aplysia californica (16.7%). Here, we identified 109 homologous unigenes of 15 decided shell matrix proteins, including nacrein, Pif, perlucin, tyrosinase (Tyr), PfN44, PUSP1, chitinase, shell matrix protein, MSI80, fibronectin type III, AmOxCo, perlwapin, BMSP, PfCHS1 and CaLP. Two other mantle transcriptomes of Pinctada margaritifera and Pinctada fucata were also analyzed to perform a biomineralization protein comparison of the three molluscan transcriptomes. All the three compared mollusks shared four proteins, including nacrein, Pif, Tyr and PfCHS1. It was also discovered that Cristaria plicata shared more biomineralization proteins with Pinctada fucata than that with Pinctada margaritifera. Our study explored a whole draft of mantle transcriptome of freshwater mussel and unraveled genes involved in the formation of shell and pearl, making it possible to identify massive novel biomineralization proteins in mollusks.




Similar content being viewed by others
References
Addadi L, Joester D, Nudelman F, Weiner S (2006) Mollusk shell formation: a source of new concepts for understanding biomineralization processes. Chem Eur J 12:980–987
Fang Z, Yan Z, Li S, Wang Q, Cao W, Xu G, Xiong X, Xie L, Zhang R (2008) Localization of calmodulin and calmodulin-like protein and their functions in biomineralization in P. fucata. Prog Nat Sci 18:405–412
Funabara D, Ohmori F, Kinoshita S, Koyama H, Mizutani S, Ota A, Osakabe Y, Nagai K, Maeyama K, Okamoto K, Kanoh S, Asakawa S, Watabe S (2014) Novel genes participating in the formation of prismatic and nacreous layers in the pearl oyster as revealed by their tissue distribution and RNA interference knockdown. PLoS One 9:e84706
Inoue N, Ishibashi R, Ishikawa T, Atsumi T, Aoki H, Komaru A (2010) Gene expression patterns and pearl formation in the Japanese pearl oyster (Pinctada fucata): a comparison of gene expression patterns between the pearl sac and mantle tissues. Aquaculture 308:S68–S74
Jiao Y, Wang H, Du X, Zhao X, Wang Q, Huang R, Deng Y (2012) Dermatopontin, a shell matrix protein gene from pearl oyster Pinctada martensii, participates in nacre formation. Biochem Biophys Res Commun 425:679–683
Joubert C, Piquemal D, Marie B, Manchon L, Pierrat F, Zanella-Cleon I, Cochennec-Laureau N, Gueguen Y, Montagnani C (2010) Transcriptome and proteome analysis of Pinctada margaritifera calcifying mantle and shell: focus on biomineralization. BMC Genom 11:1–13
Kennedy WJ, Taylor JD, Hall A (1969) Environmental and biological controls on bivalve shell mineralogy. Biol Rev Camb Philos Soc 44:499–530
Klishko OK, Lopes-Lima M, Froufe E, Bogan AE (2014) Are Cristaria herculea (Middendorff, 1847) and Cristaria plicata (Leach, 1815) (Bivalvia, Unionidae) separate species? Zookeys 438:1–15
Kong Y, Jing G, Yan Z, Li C, Gong N, Zhu F, Li D, Zhang Y, Zheng G, Wang H, Xie L, Zhang R (2009) Cloning and characterization of Prisilkin-39, a novel matrix protein serving a dual role in the prismatic layer formation from the Oyster Pinctada fucata. J Biol Chem 284:10841–10854
Li S, Xie L, Ma Z, Zhang R (2005) cDNA cloning and characterization of a novel calmodulin-like protein from pearl oyster Pinctada fucata. FEBS J 272:4899–4910
Lowenstam HA (1989) On Biomineralization. Oxford University, USA
Mann K, Jackson DJ (2014) Characterization of the pigmented shell-forming proteome of the common grove snail Cepaea nemoralis. BMC Genom 15:249
Mann K, Edsinger-Gonzales E, Mann M (2012) In-depth proteomic analysis of a mollusc shell: acid-soluble and acid-insoluble matrix of the limpet Lottia gigantea. Proteome sci 10:28
Marie B, Roy NL, Zanella-Cléon I, Becchi M, Marin F (2011) Molecular evolution of mollusc shell proteins: insights from proteomic analysis of the edible mussel mytilus. J Mol Evol 72:531–546
Marin F, Luquet G (2004) Molluscan shell proteins. CR Palevol 3:469–492
Marxen JC, Nimtz M, Becker W, Mann K (2003) The major soluble 19.6 kDa protein of the organic shell matrix of the freshwater snail Biomphalaria glabrata is an N-glycosylated dermatopontin. Biochim Biophys Acta Proteins Proteomics 1650:92–98
Miyamoto H, Miyashita T, Okushima M, Nakano S, Morita T, Matsushiro A (1996) A carbonic anhydrase from the nacreous layer in oyster pearls. Proc Natl Acad Sci USA 93:9657–9660
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628
Nagai K, Yano M, Morimoto K, Miyamoto H (2007) Tyrosinase localization in mollusc shells. Comp Biochem Physiol B Biochem Mol Biol 146:207–214
Patnaik BB, Wang TH, Kang SW, Hwang HJ, Park SY, Park EB, Chung JM, Song DK, Kim C, Kim S, Lee JS, Han YS, Park HS, Lee YS (2016) Sequencing, De Novo assembly, and annotation of the transcriptome of the endangered freshwater pearl bivalve, Cristaria plicata, provides novel insights into functional genes and marker discovery. PLoS One 11:e0148622
Samata T, Hayashi N, Kono M, Hasegawa K, Horita C, Akera S (1999) A new matrix protein family related to the nacreous layer formation of Pinctada fucata. FEBS Lett 462:225–229
Shi M, Lin Y, Xu G, Xie L, Hu X, Bao Z, Zhang R (2013a) Characterization of the Zhikong Scallop (Chlamys farreri) mantle transcriptome and identification of biomineralization-related genes. Mar Biotechnol 15:706–715
Shi Y, Yu C, Gu Z, Zhan X, Wang Y, Wang A (2013b) Characterization of the pearl oyster (Pinctada martensii) mantle transcriptome unravels biomineralization genes. Mar Biotechnol 15:175–187
Simakov O, Marletaz F, Cho S-J, Edsinger-Gonzales E, Havlak P, Hellsten U, Kuo D-H, Larsson T, Lv J, Arendt D, Savage R, Osoegawa K, de Jong P, Grimwood J, Chapman JA, Shapiro H, Aerts A, Otillar RP, Terry AY, Boore JL, Grigoriev IV, Lindberg DR, Seaver EC, Weisblat DA, Putnam NH, Rokhsar DS (2013) Insights into bilaterian evolution from three spiralian genomes. Nature 493:526–531
Suzuki M, Sakuda S, Nagasawa H (2007) Identification of chitin in the prismatic layer of the shell and a chitin synthase gene from the Japanese pearl Oyster, Pinctada fucata. Biosci Biotechnol Biochem 71:1735–1744
Suzuki M, Saruwatari K, Kogure T, Yamamoto Y, Nishimura T, Kato T, Nagasawa H (2009) An acidic matrix protein, Pif, is a key macromolecule for nacre formation. Science 325:1388–1390
Treccani L, Mann K, Heinemann F, Fritz M (2006) Perlwapin, an abalone nacre protein with three four-disulfide core (Whey acidic protein) domains, inhibits the growth of calcium carbonate crystals. Biophys J 91:2601–2608
Weiss IM, Kaufmann S, Mann K, Fritz M (2000) Purification and identification of perlucin and perlustrin, two new proteins from the shell of the mollusc Haliotis laevigata. Biophys Biochem Res Commun 267:17–21
Yan Z (2007) Biomineralization: functions of calmodulin-like protein in the shell formation of pearl oyster. Biochim Biophys Acta Gen Subjects 1770:1338–1344
Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, Yang P, Zhang L, Wang X, Qi H, Xiong Z, Que H, Xie Y, Holland PWH, Paps J, Zhu Y, Wu F, Chen Y, Wang J, Peng C, Meng J, Yang L, Liu J, Wen B, Zhang N, Huang Z, Zhu Q, Feng Y, Mount A, Hedgecock D, Xu Z, Liu Y, Domazet-Loso T, Du Y, Sun X, Zhang S, Liu B, Cheng P, Jiang X, Li J, Fan D, Wang W, Fu W, Wang T, Wang B, Zhang J, Peng Z, Li Y, Li N, Wang J, Chen M, He Y, Tan F, Song X, Zheng Q, Huang R, Yang H, Du X, Chen L, Yang M, Gaffney PM, Wang S, Luo L, She Z, Ming Y, Huang W, Zhang S, Huang B, Zhang Y, Qu T, Ni P, Miao G, Wang J, Wang Q, Steinberg CEW, Wang H, Li N, Qian L, Zhang G, Li Y, Yang H, Liu X, Wang J, Yin Y, Wang J (2012) The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490:49–54
Acknowledgements
This work is supported by the National Natural Science Foundation of China (NSFC) under Grant No. 511022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Every author declares that he has no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Communicated by S. Hohmann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Liu, Z. & Wu, W. Transcriptome analysis of the freshwater pearl mussel (Cristaria plicata) mantle unravels genes involved in the formation of shell and pearl. Mol Genet Genomics 292, 343–352 (2017). https://doi.org/10.1007/s00438-016-1278-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-016-1278-9