Abstract
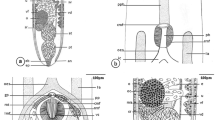
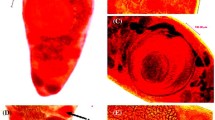
Ten adult Quinqueserialis quinqueserialis specimens were removed from the intestine of a naturally infected muskrat, and scanning electron microscopy was used to study the morphological characteristics of the trematodes. The mature trematode, which was easy to recognize by the monostome holdfast organ, with no anterior cone, measured 2200–2500 μm in length by 900–1050 μm in width. The body was elongated and tapering at the anterior end, but the posterior end was rounded, and in some specimens was slightly truncated. The mouth opening lay at the anterior end and was surrounded by the oral sucker, which was round, small to medium in size, and subterminal. The tegument of the rim and inside of the oral sucker was smooth and had two types of papillae, domed and rosette papillae. Around the oral sucker, tegument was covered with sharp, pointed spines. The common genital pore was located on the median line of the body, posterior to the oral sucker. The cirrus had smooth tegument at the base and was armed with numerous conical spines throughout its length. The ventral surface was concave and provided with five distinct longitudinal rows of ventral papillae, which extended from the anterior to the posterior end of the body. Each row consisted of 15 to 20 papillae, making 81 to 88 papillae in all. These papillae were variable in size. In most specimens, the papillae were simple knob-like structures, but in some cases, they appeared to be bi- or trifurcate. The tegument at the base of each ventral papilla showed minute spiny pattern, but it was smooth or folded on top and had small rosette and ciliated papillae. Tegument at the edges of the worm was smooth in the mid-parts, spiny on lateral parts, and included rosette papillae. The dorsal surface of the worm was smooth and slightly convex, and the tegument was provided with two large domed papillae in one third of the anterior end of the dorsal part, few thick spines in the mid-part, and excretory pore at the level just posterior to the end. No spines or papillae were seen around the excretory pore.




Similar content being viewed by others
References
Al Saadi M, Post G (1976) Rodent leptospirosis in Colorado. J Wildl Dis 12:315–317
Armstrong WH (1942) Occurrence of Salmonella typhimurium infection in muskrats. Cornell Vet 32:87–89
Awad AH, Probert AJ (1989) Transmission and scanning electron microscopy of the male reproductive system of Schistosoma margrebowiei Le Roux, 1933. J Helminthol 63(3):197–205
Bakke TA (1976a) Shape, size and surface topography of genital organs of Leucochloridium sp. (Digenea), revealed by light and scanning electron microscopy. Parasitol Res 51:99–113
Bakke TA (1976b) Functional morphology and surface topography of Leucochloridium sp. (Digenea), revealed by light and scanning electron microscopy. Parasitol Res 51:115–128
Bakke T, Hoole D (1988) The microtopography and papillar arrangement on adult Gorgoderina vitelliloba (Olsson) (Digenea, Gorgoderidae) from amphibians in relation to fish gorgoderids. Zool Scr 17(3):223–230
Barker FD, Laughlin JW (1911) A new species of trematode from the muskrat, Fiber zibethicus. Trans Am Microsc Soc 30(4):261–274
Barton DP, Blair D (2001) Family Notocotylidae Lῢhe, 1909. In: Jones A, Bray RA, Gibson DI (eds) Keys to the trematoda, vol 2. CABI, London, pp 383–396
Beckett JV, Gallicchio V (1967) A survey of helminths of the muskrat, Ondatra z. zibethica Miller, 1912, in Portage County, Ohio. J Parasitol 53(6):1169–1172
Bennet CE (1975) Scanning electron microscopy of Fasciola hepatica L. during growth and maturation in the mouse. J Parasitol 61:892–898
Beverley-Burton M, Sweeny PR (1972) Intranuclear, paracrystalline inclusions in various cells of Quinqueserialis quinqueserialis and Notocotylus urbanensis (Trematoda: Notocotylidae). Can J Zool 50(3):345–348
Beverley-Burton M, Vance H, Logan VH (1976) The ventral papillae of notocotylid trematodes. J Parasitol 62(1):148–151
Birmani NA, Dharejo AM, Khan MM (2011) Catatropis sp. (Trematoda: Notocotylidae) from the black coot, Fulicaatra Linnaeus, 1758 (Gruiformes: Rallidae) in Sindh province of Pakistan. J Animal Plant Sci 21(4):872–873
Boyce K, Hide G, Craig PS, Harris PD, Reynolds C, Pickles A, Rogan MT (2012) Identification of a new species of digenean Notocotylus malhamensis n. sp. (Digenea: Notocotylidae) from the bank vole (Myodes glareolus) and the field vole (Microtus agrestis). Parasitol 139(12):1630–1639
Chaisiri K, Morand S, Ribas A (2011) Notocotylus loeiensis n. sp. (Trematoda: Notocotylidae) from Rattus losea (Rodentia: Muridae) in Thailand. Parasite 18(1):35–38
Chalmers GA, MacNeill AC (1977) Tyzzer’s disease in wild-trapped muskrats in British Columbia. J Wildl Dis 13(114):116
Garcia-Corrales P, Cifrian B (1988) Scanning electron microscopy of adult Dicrocoelium dendriticum. Parasitol Res 74:235–242
Debbie JG (1968) Yellow fat disease in muskrats. New York Fish Game J 15:119–120
Detwiler JT, Zajac AM, Minchella DJ, Belden LK (2012) Revealing cryptic parasite diversity in a definitive host: echinostomes in muskrats. J Parasitol 98(6):1148–1155
Errington PL (1963) Muskrat populations. Iowa State Univ. Press, Ames, 665 pp
Ferrer JR, Gracenea M, Trullols M, Gonzalez-Moreno O (1996) Ultrastructural observations of the tegument of Postorchigenes gymnesicus (Digenea: Lecithodendriidae). J Helminthol 70(1):13–19
Flores V, Brugni N (2003) Catatropis chilinae n. sp. (Digenea: Notocotylidae) from Chilina dombeiana (Gastropoda: Pulmonata) and notes on its life-cycle in Patagonia, Argentina. Syst Parasitol 54(2):89–96
Flores V, Brugni N (2005) Notocotylus biomphalariae n. sp. (Digenea: Notocotylidae) from Biomphalaria peregrina (Gastropoda: Pulmonata) in Patagonia, Argentina. Syst Parasitol 61(3):207–214
Flores V, Brugni N (2006) Catatropis hatcheri n. sp. (Digenea: Notocotylidae) from Heleobia hatcheri (Prosobranchia: Hydrobiidae) and notes on its life-cycle in Patagonia, Argentina. Syst Parasitol 63(2):111–118
Fujino T, Ishii Y, Choi DW (1979) Surface ultrastructure of the tegument of Clonorchissinensis newly excysted juveniles and adult worms. J Parasitol 65:579–590
Godin AJ (1977) Wild mammals of New England. John Hopkins Univ. Press, Baltimore, 304 pp
Han ET, Han KY, Chai JY (2003) Tegumental ultrastructure of the juvenile and adult Himasthla alincia (Digenea: Echinostomatidae). Korean J Parasitol 41(1):17–25
Han ET, Choi MS, Choi SY, Chai JY (2011) Surface ultrastructure of juvenile and adult Acanthoparyphium tyosenense (Digenea: Echinostomatidae). J Parasitol 97(6):1049–1054
Harwood PD (1939) Notes on Tennessee helminthes. IV. North American trematodes of the subfamily Notocotylinae. J Tenn Acad Sci 14:421–437
Herber EC (1942) Life history studies on two trematodes of the subfamily Notocotylinae. J Parasitol 28(3):179–196
Hong SJ, Chai JY, Lee SH (1991) Surface ultrastructure of the developmental stages of Heterophyopsis continua (Trematoda: Heterophyidae). J Parasitol 77(4):613–620
Hoole D, Mitchell JB (1981) Ultrastructural observations on the sensory papillae of juvenile and adult Gorgoderina vitelliloba (Trematoda: Gorgoderidae). Int Parasitol 11:411–417
Iwaki T, Okada T, Seki K, Izawa K, Sakurai F (2012) Ogmocotyle ailuri (Price, 1954) (Digenea: Notocotylidae) found in the Japanese monkey, Macaca fuscata. J Vet Med Sci 74(9):1211–1212
Kinsella JM (1971) Growth, development, and intraspecific variation of Quinqueserialis quinqueserialis (Trematoda: Notocotylidae) in rodent hosts. J Parasitol 57(1):62–70
Kinsella JM, Tkach VV (2005) Notocotylus fosteri sp. nov. (Trematoda, Notocotylidae) from the rice rat, Oryzomys palustris in Florida. Acta Parasitologica 50(3):194–198
Kirillova NI, Kirillov AA (2009) Trematodes (Trematoda) of micromammals from Middle Volga region. Parazitologiia 43(3):225–239
Langford EV (1972) Pasteurella pseudotuberculosis infections in western Canada. Can Vet J 13:85–87
Lowery HG Jr (1974) The mammals of Louisiana and its adjacent waters. Louisiana State University Press, Baton Rouge, p 565
MacKinnon BM (1982) The development of the ventral papillae of Notocotylus triserialis (Digenea: Notocotylidae). Parasitol Res 68(3):279–293
Mata-Lopez R, Leon-Regagnon V (2006) Comparative study of the tegumental surface of several species of Gorgoderina Looss, 1902 (Digenea: Gorgoderidae), as revealed by scanning electron microscopy. Comp Parasitol 73(1):24–34
McKenzie CE, Welsh HE (1979) Parasite fauna of the muskrat, Ondatra zibethica (Linnaeus, 1766) in Manitoba, Canada. Can J Zool 57:640–646
Naem S, Budke CM, Craig TM (2012) Morphological characterization of adult Fascioloides magna (Trematoda: Fasciolidae): first SEM report. Parasitol Res 110:971–978
Namue C, Wongsawad C (1997) A survey of helminth infection in rats (Rattus spp) from Chiang Mai Moat. Southeast Asian J Trop Med Public Health 28(Suppl 1):179–183
Otcenásek M, Rosický B, Krivanec K, Dvorák J, Rasín K (1974) The muskrat as reservoir in natural foci of adiaspiromycosis. Folia Parasit 21:55–57
Otubanjo OA (1985) Scanning electron microscopic studies of the body surface and external genitalia of a dicrocoeliid trematode Concinnum epomopis Sandground 1973. Parasitol Res 71:495–504
Page MR, Nadakavukaren MJ, Huizinga HW (1980) Ribeiroia marini surface ultrastructure of redia, cercaria and adult. Int J Parasitol 10:5–12
Radlett AJ (1980) The structure and possible function of the ventral papillae of Notocotylus attenuatus (Rudolphi, 1809) Kossack, 1911, (Trematoda: Notocotylidae). Parazitologiia 80(2):241–246
Rausch RL (1952) Helminths from the round tailed muskrat, Neofiberalleni nigrescens Howell, with descriptions of two new species. J Parasitol 38(2):151–156
Santos MJ, Gibson DI (2002) Morphological features of Prosorhynchus crucibulum and P. aculeatus (Digenea: Bucephalidae), intestinal parasites of Conger conger (Pisces: Congridae), elucidated by scanning electron microscopy. Folia Parasitol 49(2):96–102
Schuster R (1986) Echinostoma echinatum, Notocotylus noyeri and Quinqueserialis quinqueserialis as unusual parasites of Rattus norvegicus. Angew Parasitol 27(4):221–225
Schuster RK (2011) Philophthalmus aweerensis n. sp. (Trematoda: Philophthalmidae) found in a rhea (Rhea americana) in the United Arab Emirates. Parasitol Res 109(4):1029–1033
Seegers G, Baumeister S, Kuschfeldt S, Pohlmeyer K, Stoye M (1997) Nematode and trematode fauna of the muskrat (Ondatra zibethicus LINK, 1795) in Lower Saxony. Dtsch Tierarztl Wochenschr 104(12):503–504
Shuteev MM (1977) Parasite fauna of the muskrat of the Verchneobskyj Bor. Parasitologiia 6:538–540
Skála V, Bulantová J, Walker AJ, Horák P (2014) Insights into the development of Notocotylus attenuatus (Digenea: Notocotylidae) in Lymnaea stagnalis: from mother sporocyst to cercariae. Parasitol Int 63(1):94–99
Skvortsov AA (1935) ZurKenntnis der Helminth fauna der Wasserratten (Arvicola terrestris L.). Vestnik Mikrobiol Epidemioli. Parazitologiia 13:317–326
Smith CF (1954) Studies on Quinqueserialis hassalli and taxonomic considerations of the species of Quinqueserialis (Trematoda: Notocotylidae). J Parasitol 40:209–215
Spakulová M, Macko JK, Berrilli F, Dezfuli BS (2002) Description of Bucephalus anguillae n. sp. (Trematoda: Bucephalidae), a parasite of the eel Anguilla anguilla (Anguillidae) from a brackish water lagoon of the Adriatic Sea. J Parasitol 88(2):382–387
Spalatin J, Iversen JO, Hanson RP (1971) Properties of a Chlamydia psittaci isolated from muskrats and snowshoe hares in Canada. Can J Microbiol 17:935–942
Threadgold LT (1975) Electron microscope studies of Fasciola hepatica. 3. Fine structure of the prostate gland. Exp Parasitol 37:117–124
Wittrock DD (1976) Structure of the cirrus tegument of Quinqueserialis quinqueserialis (Trematoda: Notocotylidae). J Parasitol 62(5):834–836
Wittrock DD (1978) Ultrastructure of the ventral papillae (Trematoda: Notocotylidae). Parasitol Res 57:145–154
Wittrock DD (1986) Histochemical and ultrastructural studies of the prostate gland of Quinqueserialis quinqueserialis (Trematoda: Notocotylidae). Trans Am Microsc Soc 105(4):365–374
Yousif F, EL Bardicy S, Tadros M, Ayoub M (2011) First Record of Catatropis indicus Srivastava (Notocotylidae) from Gabbiellas senaariensis Küster (Bithyniidae) In Egypt. Aust J Basic Appl Sci 5(9):724–728
Zhaltsanova DS, BeliakovaIu V (1986) Intermediate host of the trematode Quinqueserialis quinqueserialis (Trematoda, Notocotylidae) in the USSR and the morphology of its parthenitae and larvae. Parazitologiia 20(4):323–326
Acknowledgments
The authors thank former Hamilton College undergraduate students Alyssa Kanagaki and Calvin Johnson for the assistance with muskrat dissection and collection of trematodes. The authors express their gratitude to Professor Larry A. Arsenault, head of the electron microscope facility, Department of Pathology and Molecular Medicine, McMaster University, Canada for his assistance in providing all facilities during the study. Furthermore, the valuable help of Mr. Ernie Spitzer, chief technician, and all technicians in the electron microscope facility are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naem, S., Smythe, A.B. Tegumental ultrastructure of adult Quinqueserialis quinqueserialis (Trematoda: Notocotylidae): an intestinal parasite of muskrat (Ondatra zibethicus). Parasitol Res 114, 2473–2480 (2015). https://doi.org/10.1007/s00436-015-4444-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4444-9