Abstract
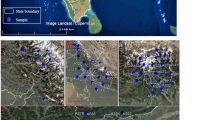
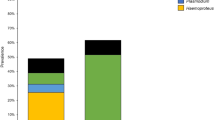
A total of 76 birds belonging to 23 species and 14 families was examined for the presence of Plasmodium spp. and Haemoproteus spp. Birds were trapped at four localities in Gansu Province, China, in June–July 2011. DNA was isolated from blood samples and parasite detection, and identification was based on PCR assays and sequences of 479 bp of cyt b gene. The total prevalence of haemosporidians was 21.0 %. Haemoproteus spp. were detected in 14 birds (prevalence 18.4 %). The lineage CYAPIC1 from Cyanopica cyanus, Parus major, Passer montanus and Pyrrhocorax pyrrhocorax was new; it is genetically distinct and probably represents a new species of the genus Haemoproteus. Three lineages represented known species: RBS4 (from Lanius tephronotus), a lineage of Haemoproteus lanii; COLL2 (from Turdus mupinensis), a lineage of Haemoproteus pallidus and TURDUS2 (from Turdus rubrocanus), a lineage of Haemoproteus minutus. The lineage RBS5 (from Lanius cristatus and L. tephronotus) differs by 1.4 % from RBS4 and probably represents an intraspecific entity of H. lanii. The lineages TUCHR1 (recorded from T. mupinensis), WW1 (recorded from Upupa epops) and YWT2 (recorded from Motacilla flava) have not been linked to any known species for the moment. Only one bird was positive for Plasmodium (prevalence 1.4 %), i.e. P. major infected with the lineage GRW4 of Plasmodium relictum. The latter lineage has been considered by previous studies as typical for migratory birds and having transmission in tropical areas only; its record in a sedentary bird in China suggests its transmission in temperate latitudes.


Similar content being viewed by others
References
Beadell JS, Gering E, Austin J, Dumbacher JP, Peirce M, Pratt TK, Atkinson CT, Fleischer RC (2004) Prevalence and differential host-specificity of two avian blood parasite genera in the Australo-Papuan region. Mol Ecol 13:3829–3844. doi:10.1111/j.1365-294×.2004.02363.x
Beadell JS, Ishtiaq F, Covas R, Melo M, Warren BH, Atkinson CT, Bensch S, Graves GR, Jhala YV, Peirce MA, Rahmani AR, Fonseca DM, Fleischer RC (2006) Global phylogeographic limits of Hawaii’s avian malaria. Proc R Soc Lond, B Biol Sci 273:2935–2944. doi:10.1098/rspb.2006.3671
Bensch S, Stjernman M, Hasselquist D, Ostman O, Hansson B, Westerdahl H, Pinheiro RT (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc R Soc Lond, B Biol Sci 267:1583–1589. doi:10.1098/rspb.2000.1181
Bensch S, Pérez-Tris J, Waldenstrom J, Hellgren O (2004) Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: multiple cases of cryptic speciation? Evolution 58:1617–1621. doi:10.1111/j.0014-3820.2004.tb01742.x
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358. doi:10.1111/j.1755-0998.2009.02692.x
Chen Y, Li G (2004) Survey of protozoan parasites of blood for decorative birds in China. China Anim Quar 2004–01
Ejiri H, Sato Y, Sasaki E, Sumiyama D, Tsuda Y, Sawabe K, Matsui S, Horie S, Akatani K, Takagi M, Omori S, Murata K, Yukawa M (2008) Detection of avian Plasmodium spp. DNA sequences from mosquitoes captured in Minami Daito Island of Japan. J Vet Med Sci 70:1205–1210
Fallon SM, Bermingham E, Ricklefs RE (2005) Host specialization and geographic localization of avian malaria parasites: a regional analysis in the Lesser Antilles. Am Nat 165:466–480. doi:10.1086/428430
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows95/98/NT. Nucleic Acids Symp Ser 41:95–98
He JG (1988) Plasmodium parajuxtanucleare sp. n. from Bambusicola thoracica in Guangdong. Annu Bull Soc Parasitol of Guangdong Province 10:158–160 (in Chinese)
He JG, Huang JC (1993) A new species of avian malaria parasite from Pycnonotus jocosus (Sporozoa: Plasmodiidae). Acta Zootaxonomica Sin 18:129–133 (in Chinese)
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium and Haemoproteus from avian blood. J Parasitol 90:797–802. doi:10.1645/GE-184R1
Huang JC (1987) Notes on Plasmodium rouxi naturally infecting avian in Guangdong. Acta Zootaxonomica Sin 12:15 (in Chinese)
Huang JC (1988) Study on Plasmodium leanucleus, a new avian malarial parasite. Annu Bull Soc Parasitol of Guangdong Province 10:153–156 (in Chinese)
Huang JC (1991a) A new report of Plasmodium dissanaikei from Pycnonotus jocosus in Guangdong, with description of the species. Annu Bull Soc Parasitol of Guangdong Province 11–13:60–61 (in Chinese)
Huang JC (1991b) A study of experimental infection and description of Plasmodium luphurae from Guangdong. Annu Bull Soc Parasitol of Guangdong Province 11–13:59–60 (in Chinese)
Huang JC (1991c) A new species of the genus Plasmodium—Plasmodium leanucleus (Eucoccidia: Plasmodiidae). Acta Zootaxonomica Sin 16:257–262 (in Chinese)
Huang JC, Chen WF (1988) Plasmodium circumflexum, a new record of avian malarial parasite from Zosterops japonica in Hainan Island. Annu Bull Soc Parasitol of Guangdong Province 10:156–157 (in Chinese)
Huang JC, He JG (1991) Six plasmodia tend to one pole or another of erythrocyte from the birds in China. Acta Scientiarum Naturalium Universitatis Sunyatseni 30:71–75 (in Chinese)
Huang JC, Huang DF (1995) A new species of the bird malarial parasite Plasmodium (Novyella) bambusicolai (Sporozoa: Plasmodiidae). Acta Zootaxonomica Sin 20:385–390 (in Chinese)
Huang JC, Wu SJ (1988) A new record of bird malarial parasite from Anthus hodgsoni in China. Annu Bull Soc Parasitol of Guangdong Province 10:157–158 (in Chinese)
Huang JC, Wu SJ (1990) Plasmodium hexamerium. Acta Scientiarum Naturalium Universitatis Sunyatseni 29:132–134 (in Chinese)
Huang JC, Huang DF, Jiang JB (1995) A new species of the genus Plasmodium, Plasmodium arachnidi from the domestic pigeons in Guangzhou. Acta Vet et Zootech Sin 26:352–357 (in Chinese)
Ishtiaq F, Gering E, Rappole JH, Rahmani AR, Jhala YV, Dove CJ, Milensky C, Olson SL, Pierce MA, Fleischer RC (2007) Prevalence and diversity of avian hematozoan parasites in Asia: a regional survey. J Wildl Dis 43:382–398
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism. Academic, New York, pp 21–132
Kim KS, Tsuda Y (2010) Seasonal changes in the feeding pattern of Culex pipiens pallens govern the transmission dynamics of multiple lineages of avian malaria parasites in Japanese wild bird community. Mol Ecol 19:5545–5554. doi:10.1111/j.1365-294×.2010.04897.x
Kim KS, Tsuda Y, Yamada A (2009) Bloodmeal identification and detection of avian malaria parasite from mosquitoes (Diptera: Culicidae) inhabiting coastal areas of Tokyo Bay. Japan J Med Entomol 46:1230–1234
Križanauskienė A, Hellgren O, Kosarev V, Sokolov L, Bensch S, Valkiūnas G (2006) Variation in host specificity between species of avian haemosporidian parasites: evidence from parasite morphology and cytochrome b gene sequences. J Parasitol 92:1319–1324. doi:10.1645/GE-873R.1
Križanauskienė A, Pérez-Tris J, Palinauskas V, Hellgren O, Bensch S, Valkiūnas G (2010) Molecular phylogenetic and morphological analysis of haemosporidian parasites (Haemosporida) in a naturally infected European songbird, the blackcap Sylvia atricapilla, with description of Haemoproteus pallidulus sp nov. Parasitology 137:217–227
Li GF, He JG, Jiang JB (2003) A new malaria parasite of Turnix tanki blanfordi [blanfordi] from Guangxi. Acta Sci Nat Univ Sunyatseni 42:74–77
McClure HE, Poonswad P, Greiner EC, Laird M (1978) Haematozoa in the birds of eastern and southern Asia. Memorial University of Newfoundland, St John’s, Canada, p 296
Paperna I, Martelli P (2008) Haemosporidian infections in captive exotic glossy starling Lamprotornis chalybaeus in Hong Kong. Folia Parasitol 55:7–12
Pérez-Tris J, Bensch S (2005) Diagnosing genetically diverse avian malaria infections using mixed-sequence analysis and TA-cloning. Parasitology 131:1–9. doi:10.1017/S003118200500733X
Perkins SL, Schall JJ (2002) A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J Parasitol 88:972–978. doi:10.1645/0022-3395(2002)088[0972:AMPOMP]2.0.CO;2
Richardson DS, Jury FL, Blaakmeer K, Komdeur J, Burke T (2001) Parentage assignment and extra-group paternity in a cooperative breeder: the Seychelles warbler (Acrocephalus sechellensis). Mol Ecol 10:2263–2273. doi:10.1046/j.0962-1083.2001.01355.x
Ricklefs RE, Fallon SM (2002) Diversification and host switching in avian malaria parasites. Proc R Soc Lond, B Biol Sci 269:885–892. doi:10.1098/rspb.2001.1940
Szymanski MM, Lovette IJ (2005) High lineage diversity and host sharing of malarial parasites in a local avian assemblage. J Parasitol 91:768–774. doi:10.1645/GE-417R1.1
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 28:2731–2739
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton, Florida
Valkiūnas G, Zehtindjiev P, Hellgren O, Ilieva M, Iezhova TA, Bensch S (2007) Linkage between mitochondrial cytochrome b lineages and morphospecies of two avian malaria parasites, with a description of Plasmodium (Novyella) ashfordi sp. nov. Parasitol Res 100:1311–1322
Valkiūnas G, Iezhova T, Križanauskienė A, Palinauskas V, Sehgal RNM, Bensch S (2008) A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J Parasitol 94:1395–1401
Waldenström J, Bensch S, Kiboi D, Hasselquist D, Ottosson U (2002) Cross-species infection of blood parasites between resident and migratory songbirds in Africa. Mol Ecol 11:1545–1554. doi:10.1046/j.1365-294×.2002.01523.x
Waldenström J, Bensch S, Hasselquist D, Östman Ö (2004) A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J Parasitol 90:191–194. doi:10.1645/GE-3221RN
Zhu QS, Huang JC, Zuo YX (2012) A new malaria species: Plasmodium lanius n. sp. Jishengchong Yu Yixue Kunchong Xueba 19:172–175
Acknowledgments
We are grateful to Professor Kuizheng Cai, Professor Jialin Bai and the students Chuanshi Yan, Yuzhuo Xu, Janchiao Liu and Cuilan Wu (College of Life Science and Engineering, Northwest University for Nationalities, Lanzhou, People’s Republic of China) for their assistance in the field studies. The field trip of JM and BBG in Gansu Province was in the frames of a project supported by the National Science Foundation, USA (PBI awards no. 0818696 to Professor Janine N. Caira and no. 0818823 to Professor Kirsten Jensen). PZ was partly funded by the project WETLANET (FP7, Capacities, REGPOT, grant 229802). The present study used facilities developed at the Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, in the frames of the project CEBDER funded by Bulgarian National Science Fund, grant DOO2-15/17.02.2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zehtindjiev, P., Ivanova, K., Mariaux, J. et al. First data on the genetic diversity of avian haemosporidians in China: cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Gansu Province. Parasitol Res 112, 3509–3515 (2013). https://doi.org/10.1007/s00436-013-3533-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3533-x