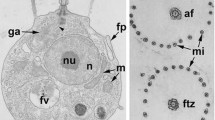
Abstract
The problem of the origin of Metazoa has been one of the most discussed for nearly the last one and a half centuries. Some 20 years ago, morphological approaches were replaced with molecules, but the problem then became more complex. At the same time, morphological data were incomplete, and therefore, this approach can still help in comparison with choanoflagellates and sponges—two sister groups having uniflagellated cells with a collar. The structure of the flagellar apparatus has phylogenetic significance, but sponge choanocytes are poorly studied in this respect, and we still do not know what the ancestral kinetid of Porifera looks like. The kinetid structure of choanocytes in Sycon sp. is investigated here for the first time, and a 3D reconstruction of the kinetid provided. It is composed of a flagellar kinetosome with a nuclear fibrillar root and a basal foot with a few microtubules; the accessory centriole lies orthogonal to and just below the foot of the kinetosome, and a dictyosome is near the centriole. This kinetid is similar to that of the choanocyte of Corticium candelabrum (Homoscleromorpha) and is considered to be the ancestral type for the whole branch Calcarea + Homoscleromorpha.



Similar content being viewed by others
References
Adl SM, Simpson AGB, Farmer M et al (2005) The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J Eukaryot Microbiol 52:399–451
Adl SM, Simpson AGB, Lane CE et al (2012) The Revised Classification of Eukaryotes. J Eukaryot Microbiol 59:429–493
Amano S, Hori I (2001) Metamorphosis of coeloblastula performed by multipotential larval flagellated cells in the calcareous sponge Leucosolenia laxa. Biol Bull 200:20–32
Borchiellini C, Chombard C, Manuel M, Alivon E, Vacelet J, Boury-Esnault N (2004) Molecular phylogeny of Demospongiae: implications for classification and scenarios of character evolution. Mol Phylogenet Evol 32:823–837
Boury-Esnault N, De Vos L, Donadey C, Vacelet J (1984) Comparative study of the choanosome of Porifera I. the homoscleromorpha. J Morphol 180:3–17
Brill B (1973) Untersuchungen zur Ultrastruktur der Choanocyte von Ephydatia fluviatilis L. Z Zellforsch 144:231–245
Dohrmann M, Voigt O, Erpenbeck D, Wörheide G et al (2006) Non-monophyly of most supraspecific taxa of calcareous sponges (Porifera, Calcarea) revealed by increased taxon sampling and partitioned Bayesian analysis of ribosomal DNA. Mol Phylogenet Evol 40:830–843
Eerkes-Medrano DI, Leys SP (2006) Ultrastructure and embryonic development of a syconoid calcareous sponge. Invert Biol 125:177–194
Erpenbeck D, Wörheide G (2007) On the molecular phylogeny of sponges (Porifera). Zootaxa 1668:107–126
Efremova SM, Sukhodolskaya AN, Alekseeva NP (1988) The different structure of kinetosome rootlet systems in flagellated cells of the larvae and the choanocytes of sponges. In: Koltun BM, Stepaniants CD (eds). Porifera and Cnidaria. Modern and Perspective Investigations. Leningrad: USSR Academy of Sciences, Zoological institute. pp 22–23 (in Russian)
Ereskovsky AV, Tokina DB, Bezac C, Boury-Esnault N (2007) Metamorphosis of Cinctoblastula Larvae (Homoscleromorpha, Porifera). J Morph 268:518–528
Gonobobleva E, Maldonado M (2009) Choanocyte Ultrastructure in Halisarca dujardini (Demospongiae, Halisarcida). J Morph 270:615–627
Hartman WD, Willenz P (1990) Organization of the choanosome of three Caribbean sclerosponges. In: Rützler K (ed) New perspectives in sponge biology. Smithsonian Institution Press, Washington, pp 228–236
James-Clark H (1868) On the spongia ciliata as Infusoria flagellata. Ann Mag Nat Hist 1:133–142, 188–215, 250–264
Karpov SA (2000) Flagellate phylogeny: ultrastructural approach. In: Leadbeater BSC, Green JC (eds) The flagellates. Systematics Association Special Publications London, Taylor & Francis, pp 336–360
Karpov SA, Efremova SM (1994) Ultrathin structure of the flagellar apparatus in the choanocyte of the sponge Ephydatia fluviatilis. Tsitologia 36:403–408 (in Russian)
Karpov SA, Leadbeater BSC (1998) Cytoskeleton structure and composition in choanoflagellates. J Eukaryot Microbiol 45:361–367
Kilian EF (1954) Die feinstruktur des kragen bei den choanocyten der spongilliden. Ber Oberhess Gesellesch Natur Heilk Giessen. N F Naturw Abt 27:85–89
King N (2004) The unicellular ancestry of animal development. Dev Cell 7:313–325
Maldonado M (2004) Choanoflagellates, choanocytes, and animal multicellularity. Invert Biol 123:1–22
Maldonado M (2009) Embryonic development of verongid demosponges supports the independent acquisition of spongin skeletons as an alternative to the siliceous skeleton of sponges. Biol J Linn Soc 97:427–447
Manuel M (2006) Phylogeny and evolution of calcareous sponges. Can J Zool 84:225–241
Manuel M, Borchiellini C, Alivon E, Le Parco Y, Vacelet J, Boury-Esnault N (2003) Phylogeny and evolution of calcareous sponges: monophyly of Calcinea and Calcaronea, high level of morphological homoplasy, and the primitive nature of axial symmetry. Syst Biol 52:311–333
Mehl D, Reiswig HM (1991) The presence of flagellar vanes in choanomeres of Porifera and their possible phylogenetic implications. Z Zool Syst Evol Forsch 29:312–319
Muricy G, Bézac C, Gallissian M-F, Boury-Esnault N (1999) Anatomy, cytology and symbiotic bacteria of four Mediterranean species of Plakina Schulze, 1880 (Demospongiae, Homosclerophorida). J Nat Hist 33:159–176
Philippe H, Derelle R, Lopez P et al (2009) Phylogenomics revives traditional views on deep animal relationships. Curr Biol 19:706–712
Rasmont R (1959) L’ultrastructure des choanocytes d’éponges. Ann Sci Nat Zool 12:253–262
Simpson TL (1984) The cell biology of sponges. Springer-Verlag, New York
Vacelet J, Boury-Esnault N, De Vos L, Donadey C (1989) Comparative study of the choanosome of Porifera: the keratose sponges. J Morphol 201:119–129
Watanabe Y (1978) Structure and formation of the collar in choanocytes of Tetilla serica (Lebwohl), Demospongiae. Dev Growth Differ 38:71–74
Willmer P (1991) Invertebrate Relationships. Cambridge, Cambridge University Press, Patterns in Animal Evolution
Woollacott RM, Pinto RL (1995) Flagellar basal apparatus and its utility in phylogenetic analyses of the Porifera. J Morph 226:247–265
Acknowledgments
The authors thank I. A. Tikhomirov for providing the material (adult Sycon) from the marine aquarium; S. M. Efremova and E. L. Gonobobleva for discussion on sponge histology and cytology, methods of fixation for EM; A. A. Kobzeva for some methodological recommendation. We also thank the laboratory of Electron Microscopy ZIN RAS and the Research Resource Center for Molecular and Cell Technologies (RRC MCT) at St. Petersburg State University (SPbSU) for access to the EM facilities. A project was partly supported by the RAS Presidium program “Problems of life origin and biosphere development.” We are grateful to reviewers, for the comments and improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Schmidt-Rhaesa.
Rights and permissions
About this article
Cite this article
Pozdnyakov, I.R., Karpov, S.A. Flagellar apparatus structure of choanocyte in Sycon sp. and its significance for phylogeny of Porifera. Zoomorphology 132, 351–357 (2013). https://doi.org/10.1007/s00435-013-0193-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-013-0193-4