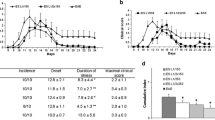
Abstract
The parasitic nematode, Trichinella spiralis (T. spiralis), exerts an immunomodulatory effect on the host immune response through excretory–secretory products (ES L1) released from encysted muscle larvae. Our model of combined T. spiralis infection and experimental autoimmune encephalomyelitis (EAE) in Dark Agouti (DA) rats demonstrated a significant reduction in EAE severity in infected animals. Recently, we have created an immune status characteristic for the live infection by in vivo application of dendritic cells (DCs) stimulated with ES L1 products of T. spiralis muscle larvae. Moreover, these cells were able to ameliorate EAE when applied 7 days before EAE induction. ES L1-stimulated DCs increased production of IL-4, IL-10 and TGF-β, and decreased production of IFN-γ and IL-17, both at the systemic level and in target organs. A significant increase in the proportion of CD4+CD25+Foxp3+ T cells was found among spleen cells, and CNS infiltrates from DA rats treated with ES L1-stimulated DCs before EAE induction, compared to controls injected with unstimulated DCs. Regulatory T cells, together with elevated levels of IL-10 and TGF-β, are most likely involved in restraining the production of Th1 and Th17 cytokines responsible for autoimmunity and thus are responsible for the beneficial effect of ES L1-educated DCs on the course of EAE. Our results show that ES L1 antigen-stimulated DCs are able not only to provoke, but also to sustain anti-inflammatory and regulatory responses regardless of EAE induction, with subsequent amelioration of EAE, or even protection from the disease.







Similar content being viewed by others
References
Rook GAW (2010) 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clinic Exp Immunol. 160:70–79
Rook GAW (2012) Hygiene hypothesis and autoimmune diseases. Clinic Rev Allerg Immunol. 42:5–15
Youinou P, Pers JO, Gershwin ME, Shoenfeld Y (2010) Geo-epidemiology and autoimmunity. J Autoimmun 34:J163–J167
Kivity S, Agmon-Levin N, Blank M, Shoenfeld Y (2009) Infections and autoimmunity—friends or foes? Trends Immunol 30:409–414
Yazdanbakhsh M, Kremsner PG, van Ree R (2002) Allergy, parasites, and the hygiene hypothesis. Science 296:490–494
Fleming JO, Cook TD (2006) Multiple sclerosis and the hygiene hypothesis. Neurology 67:2085–2086
Correale J, Farez M (2007) Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 61:97–108
Maizels RM, Yazdanbakhsh M (2008) T-cell regulation in helminth parasite infections: implications for inflammatory diseases. Chem Immunol Allergy 94:112–123
Correale J, Farez M (2011) The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol 233:6–11
Summers RW, Elliott DE, Urban JF Jr et al (2005) Trichuris suis therapy in Crohn’s disease. Gut 54:87–90
Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M et al (2008) Trichinella spiralis: modulation of experimental autoimmune encephalomyelitis in DA rats. Exp Parasitol 188:641–647
Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M et al (2010) Mechanisms of modulation of experimental autoimmune encephalomyelitis by Trichinella spiralis infection in Dark Agouti rats. Parasite Immunol 32:450–459
Sakaguchi S, Takahashi T, Yamazaki S et al (2001) Immunologic self-tolerance maintained by T-cell-mediated control of self-reactive T cells: implications for autoimmunity and tumor immunity. Microbes Infect 3:911–918
El-Malky M, Nabih N, Heder M et al (2011) Helminth infections: therapeutic potential in autoimmune disorders. Parasite Immunol 33:589–593
Fleming J, Isaak A, Lee JE et al (2011) Probiotic helminth administration in relapsing—remitting multiple sclerosis: a phase 1 study. Mult Scler. 17:743–754
Summers RW, Elliot DE, Urban JF Jr et al (2005) Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 128:825–832
McKay DM (2009) The therapeutic helminth? Trends Parasitol. 25:109–114
Wu Z, Sofronic-Milosavljevic Lj, Nagano I, Takahashi Y (2008) Trichinella spiralis: nurse cell formation with emphasis on analogy to muscle cell repair. Parasite Vectors 19:27–40
Despommier DD (1998) How does Trichinella spiralis make itself at home? Parasitol Today 14:318–323
Banchereau J, Briere F, Caux C et al (2000) Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811
Steinman RM, Hawiger D, Nussenzweig MC (2003) Tolerogenic dendritic cells. Annu Rev Immunol 21:685–711
Ilic N, Colic M, Gruden-Movsesijan A et al (2008) Characterization of rat bone marrow dendritic cells initially primed by Trichinella spiralis antigens. Parasite Immunol 30:491–495
Gruden-Movsesijan A, Ilic N, Colic M et al (2011) The impact of Trichinella spiralis excretory–secretory products on dendritic cells. Comp Immunol Microbio Infect Dis. 34:429–439
Gamble HR, Bessonov AS, Cuperlovic K et al (2000) International commission on Trichinellosis: recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet Parasitol 93:393–408
Ilic N, Worthington JJ, Gruden-Movsesijan A et al (2011) Trichinella spiralis antigens prime mixed Th1/Th2 response but do not induce de novo generation of Foxp3+ T cells in vitro. Parasite Immunol 33:572–582
Talmor M, Mirza A, Turly S et al (1998) Generation of large numbers of immature and mature dendritic cells from rat bone marrow cultures. Eur J Immunol 28:811–817
Momcilovic M, Miljkovic Z, Popadic D et al (2008) Kinetics of IFN-γ and IL-17 expression and production in active experimental autoimmune encephalomyelitis in Dark Agouti rats. Neurosci Lett 447:148–152
Hawiger D, Inaba K, Dorsett Y et al (2001) Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 194:769–779
Lutz M, Schuler G (2002) Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol 23:445–449
Lutz MB, Kurts C (2009) Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur J Immunol 39:2325–2330
Perona-Wright G, Jenkins SJ, MacDonald AS (2006) Dendritic cell activation and function in response to Schistosoma mansoni. Int J Parsitol. 36:711–721
Balic A, Harcus Y, Holland MJ, Maizels RM (2004) Selective maturation of dendritic cells by Nippostrongylus brasiliensis-secreted proteins drives Th2 immune responses. Eur J Immunol 34:3047–3059
Dowling DJ, Noone CM, Adams PN et al (2011) Ascaris lumbricoides pseudocoelomic body fluid induces a partially activated dendritic cell phenotype with Th2 promoting ability in vivo. Int J Parasitol 41:255–261
Manickasingham SP, Edwards AD, Schulc O, Reis e Sousa C (2003) The ability of murine dendritic cell subsets to direct T helper cell differentiation is dependent on microbial signals. Eur J Immunol 33:101–107
Maldonado-Lopez R, Maliszewski C, Urbain J, Moser M (2001) Cytokines regulate the capacity of CD8+ and CD8− dendritic cells to prime Th1/Th2 cells in vivo. J Immunol. 167:4345–4350
Corinti S, Albanes C, la Sala A et al (2001) Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 166:4312–4318
Hilkens CMU, Isaacs JD, Thomson AW (2010) Development of dendritic cell-based immunotherapy for autoimmunity. Int Rev Immunol 29:156–183
Lan YY, Wang Z, Raimondi G et al (2006) “Alternatively activated” dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 177:5868–5877
Stromnes IM, Cerretti LM, Liggit D et al (2008) Differential regulation of central nervous system autoimmunity by Th1 and Th17 cells. Nat Med 14:337–342
Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 133:775–787
Zhang X, Koldzic DN, Izikson L et al (2004) IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol 16:249–256
Marie JC, Letterio JJ, Gavin M, Rudensky AY (2005) TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med 201:1061–1067
Huang YM, Yang J, Xu LY et al (2000) Autoantigen-pulsed dendritic cells induce tolerance to experimental allergic encephalomyelitis (EAE) in Lewis rats. Clin Exp Immunol 122:437–444
Menges M, Rößner S, Voigtländer C et al (2002) Repetitive injections of dendritic cells matured with tumor necrosis factor-α induce antigen-specific protection of mice from autoimmunity. J Exp Med 195:15–21
Hu J, Wan Y (2011) Tolerogenic dendritic cells and their potential applications. Immunology 132:307–314
Maloy KJ, Powrie F (2001) Regulatory T cells in the control of immune pathology. Nat Immunol 2:816–822
Thornton AM, Shevach EM (2000) Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 164:183–190
Van de Keere F, Tonegawa S (1998) CD4+ T cells prevent spontaneous Experimental autoimmune encephalomyelitis in anti-myelin basic protein T cell receptor transgenic mice. J Exp Med 188:1875–1882
Acknowledgments
We are grateful to Prof. Dr. Miodrag Colic and Dr. Sergej Tomic (Military Medical Academy School of Medicine, Belgrade) for valuable assistance and helpful discussions. We also wish to express sincere thanks to Dr. Marija Mostarica-Stojkovic (Institute of Microbiology and Immunology, School of Medicine, University of Belgrade) for critical reading of the paper and providing valuable advices. This work was supported by the Ministry of Education and Science, Republic of Serbia (Project 173047).
Conflict of interest
The authors confirm that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sofronic-Milosavljevic, L., Radovic, I., Ilic, N. et al. Application of dendritic cells stimulated with Trichinella spiralis excretory–secretory antigens alleviates experimental autoimmune encephalomyelitis. Med Microbiol Immunol 202, 239–249 (2013). https://doi.org/10.1007/s00430-012-0286-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-012-0286-6