Abstract
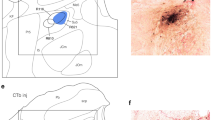
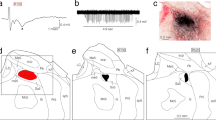
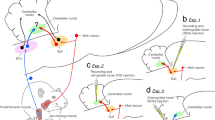
The ascending pathway mediating proprioception from the orofacial region is still not fully known. The present study elucidated the relay of jaw-closing muscle spindle (JCMS) inputs from brainstem to thalamus in rats. We injected an anterograde tracer into the electrophysiologically identified supratrigeminal nucleus (Su5), known to receive JCMS input. Many thalamic axon terminals were labeled and were found mainly contralaterally in a small, unpredicted area of the caudo-ventromedial edge (VPMcvm) of ventral posteromedial thalamic nucleus (VPM). Electrical stimulation of the masseter nerve and passive jaw movements induced large responses in the VPMcvm. The VPMcvm is far from the rostrodorsal part of ventral posterolateral thalamic nucleus (VPL) where proprioceptive inputs from the body are represented. After injection of a retrograde tracer into the electrophysiologically identified VPMcvm, many neurons were labeled almost exclusively in the contralateral Su5, whereas no labeled neurons were found in the principal sensory trigeminal nucleus (Pr5) and spinal trigeminal nucleus (Sp5). In contrast, after injection of a retrograde tracer into the core of VPM, many neurons were labeled contralaterally in the Pr5 and Sp5, but none in the Su5. We conclude that JCMS input excites trigeminothalamic projection neurons in the Su5 which project primarily to the VPMcvm in marked contrast to other proprioceptors and sensory receptors in the orofacial region which project to the core VPM. These findings suggest that lesions or deep brain stimulation in the human equivalent of VPMcvm may be useful for treatment of movement disorders (e.g., orofacial tremor) without affecting other sensations.







Similar content being viewed by others
Abbreviations
- 3V:
-
Third ventricle
- 7n:
-
Facial nerve
- 10:
-
Dorsal motor nucleus of vagus
- 12:
-
Hypoglossal nucleus
- ABC:
-
Avidin-biotin-peroxidase complex
- APT:
-
Anterior pretectal nucleus
- AVM:
-
Area ventral to the Mo5 and medial to the Pr5
- BDA:
-
Biotinylated dextranamine
- CL:
-
Centrolateral thalamic nucleus
- CM:
-
Central medial thalamic nucleus
- contra:
-
Contralateral
- Cu:
-
Cuneate nucleus
- DAB:
-
Diaminobenzidine
- DBS:
-
Deep brain stimulation
- FG:
-
Fluorogold
- fr:
-
Fasciculus retroflexus
- Gr:
-
Gracile nucleus
- HRP:
-
Horseradish peroxidase
- I5:
-
Intertrigeminal region
- IMD:
-
Intermediodorsal thalamic nucleus
- ipsi:
-
Ipsilateral
- JCMS:
-
Jaw-closing muscle spindle
- KF:
-
Kölliker–Fuse nucleus
- LHb:
-
Lateral habenular nucleus
- LPB:
-
Lateral parabrachial nucleus
- m5:
-
Trigeminal motor nerve
- Me5:
-
Mesencephalic trigeminal nucleus
- me5:
-
Mesencephalic trigeminal tract
- MD:
-
Mediodorsal thalamic nucleus
- MHb:
-
Medial habenular nucleus
- ml:
-
Medial lemniscus
- Mo5:
-
Trigeminal motor nucleus
- MPB:
-
Medial parabrachial nucleus
- PB:
-
Parabrachial nucleus
- PC:
-
Paracentral thalamic nucleus
- PF:
-
Parafascicular thalamic nucleus
- PhB:
-
Phosphate buffer
- PhBS:
-
Phosphate buffered saline
- Po:
-
Posterior thalamic nucleus
- Pr5:
-
Principal sensory trigeminal nucleus
- PV:
-
Paraventricular thalamic nucleus
- scp:
-
Superior cerebellar peduncle
- SO:
-
Superior olive
- Sol:
-
Nucleus of the solitary tract
- Sp5:
-
Spinal trigeminal nucleus
- sp5:
-
Spinal trigeminal tract
- Sp5C:
-
Sp5, caudal part
- Sp5I:
-
Sp5, interpolar part
- Sp5O:
-
Sp5, oral part
- Su5:
-
Supratrigeminal nucleus
- TMB:
-
Tetramethyl benzidine
- Vim:
-
Ventral intermediate thalamic nucleus
- VM:
-
Ventromedial thalamic nucleus
- VPL:
-
Ventral posterolateral thalamic nucleus
- VPLo:
-
Oral part of the VPL
- VPM:
-
Ventral posteromedial thalamic nucleus
- VPMcvm:
-
Caudo-ventromedial edge of the VPM
- VPPC:
-
Parvicellular part of ventral posterior thalamic nucleus
- WGA-HRP:
-
Wheat-germ agglutinin conjugated horseradish peroxidase
References
Akhter F, Haque T, Sato F, Kato T, Ohara H, Fujio T, Tsutsumi K, Uchino K, Sessle BJ, Yoshida A (2014) Projections from the dorsal peduncular cortex to the trigeminal subnucleus caudalis (medullary dorsal horn) and other lower brainstem areas in rats. Neuroscience 266:23–37
Andersson SA, Landgren S, Wolsk D (1966) The thalamic relay and cortical projection of group I muscle afferents from the forelimb of the cat. J Physiol (Lond) 183:576–591
Bolton PS, Tracey DJ (1992) Neurons in the dorsal column nuclei of the rat respond to stimulation of neck mechanoreceptors and project to the thalamus. Brain Res 595:175–179
Bruce LL, McHaffie JG, Stein BE (1987) The organization of trigeminotectal and trigeminothalamic neurons in rodents: a double-labeling study with fluorescent dyes. J Comp Neurol 262:315–330
Capra NF (1987) Localization and central projections of primary afferent neurons that innervate the temporomandibular joint in cats. Somatosens Res 4:201–213
Cechetto DF, Saper CB (1987) Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol 262:27–45
Chiaia NL, Rhoades RW, Bennett-Clarke CA, Fish SE, Killackey HP (1991a) Thalamic processing of vibrissal information in the rat. I. Afferent input to the medial ventral posterior and posterior nuclei. J Comp Neurol 314:201–216
Chiaia NL, Rhoades RW, Fish SE, Killackey HP (1991b) Thalamic processing of vibrissal information in the rat: II. Morphological and functional properties of medial ventral posterior nucleus and posterior nucleus neurons. J Comp Neurol 314:217–236
Clark GT, Koyano K, Browne PA (1993) Oral motor disorders in humans. J Calif Dent Assoc 21:19–30
Cody FW, Lee RW, Taylor A (1972) A functional analysis of the components of the mesencephalic nucleus of the fifth nerve in the cat. J Physiol (Lond) 226:249–261
Dado RJ, Giesler GJ Jr (1990) Afferent input to nucleus submedius in rats: retrograde labeling of neurons in the spinal cord and caudal medulla. J Neurosci 10:2672–2686
Dessem D, Moritani M, Ambalavanar R (2007) Nociceptive craniofacial muscle primary afferent neurons synapse in both the rostral and caudal brain stem. J Neurophysiol 98:214–223
Diamond ME, Armstrong-James M, Budway MJ, Ebner FF (1992a) Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus: dependence on the barrel field cortex. J Comp Neurol 319:66–84
Diamond ME, Armstrong-James M, Ebner FF (1992b) Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. J Comp Neurol 318:462–476
Dubner R, Sessle BJ, Storey AT (1978) The neural basis of oral and facial function. Plenum Press, New York
Erer S, Jankovic J (2007) Hereditary chin tremor in Parkinson’s disease. Clin Neurol Neurosurg 109:784–785
Fabri M, Burton H (1991) Topography of connections between primary somatosensory cortex and posterior complex in rat: a multiple fluorescent tracer study. Brain Res 538:351–357
Francis JT, Xu S, Chapin JK (2008) Proprioceptive and cutaneous representations in the rat ventral posterolateral thalamus. J Neurophysiol 99:2291–2304
Friedman DP, Jones EG (1981) Thalamic input to areas 3a and 2 in monkeys. J Neurophysiol 45:59–85
Fujio T, Sato F, Tachibana Y, Kato T, Tomita A, Higashiyama K, Ono T, Maeda Y, Yoshida A (2016) Revisiting the supratrigeminal nucleus in the rat. Neuroscience 324:307–320
Fukushima T, Kerr FW (1979) Organization of trigeminothalamic tracts and other thalamic afferent systems of the brainstem in the rat: presence of gelatinosa neurons with thalamic connections. J Comp Neurol 183:169–184
Groenewegen HJ, Witter MP (2004) Thalamus. In: Paxinos G (ed) The rat nervous system. Elsevier Academic Press, Amsterdam, pp 407–453
Hamani C, Dostrovsky JO, Lozano AM (2006) The motor thalamus in neurosurgery. Neurosurgery 58:146–158
Jones EG, Friedman DP (1982) Projection pattern of functional components of thalamic ventrobasal complex on monkey somatosensory cortex. J Neurophysiol 48:521–544
Jones EG, Friedman DP, Hendry SH (1982) Thalamic basis of place- and modality-specific columns in monkey somatosensory cortex: a correlative anatomical and physiological study. J Neurophysiol 48:545–568
Karp BI, Alter K (2016) Botulinum toxin treatment of blepharospasm, orofacial/oromandibular dystonia, and hemifacial spasm. Semin Neurol 36:84–91
Kemplay S, Webster KE (1989) A quantitative study of the projections of the gracile, cuneate and trigeminal nuclei and of the medullary reticular formation to the thalamus in the rat. Neuroscience 32:153–167
Krout KE, Loewy AD (2000) Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol 428:475–494
Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR, Murphy JT, Lenz YE (1990) Single unit analysis of the human ventral thalamic nuclear group. Activity correlated with movement. Brain 113:1795–1821
Lenz FA, Kwan HC, Martin R, Tasker R, Richardson RT, Dostrovsky JO (1994) Characteristics of somatotopic organization and spontaneous neuronal activity in the region of the thalamic principal sensory nucleus in patients with spinal cord transection. J Neurophysiol 72:1570–1587
Leong SK, Tan CK (1987) Central projection of rat sciatic nerve fibres as revealed by Ricinus communis agglutinin and horseradish peroxidase tracers. J Anat 154:15–26
Lorente de Nó R (1922) Contribución a1 conocimiento del nervio trigémino. Libro en honor de Dn. S. Ramón y Cajal. Móya, Madrid, 2:13
Lorente de Nó R (1933) Vestibulo-ocular reflex arc. Arch Neurol Psychiatr 30:245–291
Low JS, Mantle-St John LA, Tracey DJ (1986) Nucleus z in the rat: spinal afferents from collaterals of dorsal spinocerebellar tract neurons. J Comp Neurol 243:510–526
Lund JP, Richmond FJ, Touloumis C, Patry Y, Lamarre Y (1978) The distribution of Golgi tendon organs and muscle spindles in masseter and temporalis muscles of the cat. Neuroscience 3:259–270
Luo P, Dessem D (1995) Inputs from identified jaw-muscle spindle afferents to trigeminothalamic neurons in the rat: a double-labeling study using retrograde HRP and intracellular biotinamide. J Comp Neurol 353:50–66
Luo P, Wong R, Dessem D (1995) Ultrastructural basis for synaptic transmission between jaw-muscle spindle afferents and trigeminothalamic neurons in the rostral trigeminal sensory nuclei of the rat. J Comp Neurol 363:109–128
Luo P, Moritani M, Dessem D (2001) Jaw-muscle spindle afferent pathways to the trigeminal motor nucleus in the rat. J Comp Neurol 435:341–353
Maendly R, Rüegg DG, Wiesendanger M, Wiesendanger R, Lagowska J, Hess B (1981) Thalamic relay for group I muscle afferents of forelimb nerves in the monkey. J Neurophysiol 46:901–917
Marfurt CF (1981) The central projections of trigeminal primary afferent neurons in the cat as determined by the tranganglionic transport of horseradish peroxidase. J Comp Neurol 203:785–798
Mesulam MM (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 126:106–117
Narabayashi H, Ohye C (1980) Importance of microstereoencephalotomy for tremor alleviation. Appl Neurophysiol 43:222–227
Norgren R, Leonard CM (1973) Ascending central gustatory pathways. J Comp Neurol 150:217–237
Ohya A (1992) Responses of trigeminal subnucleus interpolaris neurons to afferent inputs from deep oral structures. Brain Res Bull 29:773–781
Ohya A, Tsuruoka M, Imai E, Fukunaga H, Shinya A, Furuya R, Kawawa T, Matsui Y (1993) Thalamic- and cerebellar-projecting interpolaris neuron responses to afferent inputs. Brain Res Bull 32:615–621
Ohye C (2000) Use of selective thalamotomy for various kinds of movement disorder, based on basic studies. Stereotact Funct Neurosurg 75:54–65
Ohye C, Shibazaki T, Hirai T, Wada H, Hirato M, Kawashima Y (1989) Further physiological observations on the ventralis intermedius neurons in the human thalamus. J Neurophysiol 61:488–500
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, Sydney
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic Press, Sydney
Paxinos G, Watson C (2014) The rat brain in stereotaxic coordinates, 7th edn. Academic Press, Sydney
Pierret T, Lavallée P, Deschênes M (2000) Parallel streams for the relay of vibrissal information through thalamic barreloids. J Neurosci 20:7455–7462
Romfh JH, Capra NF, Gatipon GB (1979) Trigeminal nerve and temporomandibular joint of the cat: a horseradish peroxidase study. Exp Neurol 65:99–106
Sato F, Akhter F, Haque T, Kato T, Takeda R, Nagase Y, Sessle BJ, Yoshida A (2013) Projections from the insular cortex to painreceptive trigeminal caudal subnucleus (medullary dorsal horn) and other lower brainstem areas in rats. Neuroscience 233:9–27
Shigenaga Y, Nakatani Z, Nishimori T, Suemune S, Kuroda R, Matano S (1983) The cells of origin of cat trigeminothalamic projections: especially in the caudal medulla. Brain Res 277:201–222
Takemura M, Sugimoto T, Shigenaga Y (1991) Difference in central projection of primary afferents innervating facial and intraoral structures in the rat. Exp Neurol 111:324–331
Taylor A (1990) Neurophysiology of the jaws and teeth. Macmillan Press, London
Veinante P, Deschênes M (1999) Single- and multi-whisker channels in the ascending projections from the principal trigeminal nucleus in the rat. J Neurosci 19:5085–5095
Wolraich D, Vasile Marchis-Crisan C, Redding N, Khella SL, Mirza N (2010) Laryngeal tremor: co-occurrence with other movement disorders. ORL J Otorhinolaryngol Relat Spec 72:291–294
Yasui Y, Saper CB, Cechetto DF (1989) Calcitonin gene-related peptide immunoreactivity in the visceral sensory cortex, thalamus, and related pathways in the rat. J Comp Neurol 290:487–501
Yoshida A, Dostrovsky JO, Sessle BJ, Chiang CY (1991) Trigeminal projections to the nucleus submedius of the thalamus in the rat. J Comp Neurol 307:609–625
Yoshida A, Dostrovsky JO, Chiang CY (1992) The afferent and efferent connections of the nucleus submedius in the rat. J Comp Neurol 324:115–133
Yoshida A, Taki I, Chang Z, Iida C, Haque T, Tomita A, Seki S, Yamamoto S, Masuda Y, Moritani M, Shigenaga Y (2009) Corticofugal projections to trigeminal motoneurons innervating antagonistic jaw muscles in rats as demonstrated by anterograde and retrograde tract tracing. J Comp Neurol 514:368–386
Acknowledgements
We are indebted to Dr. Akiko Tomita, Dr. Yume Uemura, Ms. Etsuko Ikenoue and Ms. Yumi Tsutsumi for their technical help. This work was supported by Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant 26293391 and 16K15775 to A.Y. and Grant 15H06387 to O.H.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Rights and permissions
About this article
Cite this article
Yoshida, A., Fujio, T., Sato, F. et al. Orofacial proprioceptive thalamus of the rat. Brain Struct Funct 222, 2655–2669 (2017). https://doi.org/10.1007/s00429-016-1363-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-016-1363-1