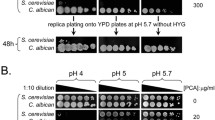
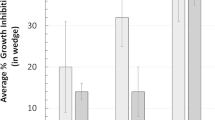
Abstract
Main conclusion
Genome-wide screening of Saccharomyces cerevisiae revealed that signaling pathways related to the alkaline pH stress contribute to resistance to plant antimicrobial peptide, Pn-AMP1.
Plant antimicrobial peptides (AMPs) are considered to be promising candidates for controlling phytopathogens. Pn-AMP1 is a hevein-type plant AMP that shows potent and broad-spectrum antifungal activity. Genome-wide chemogenomic screening was performed using heterozygous and homozygous diploid deletion pools of Saccharomyces cerevisiae as a chemogenetic model system to identify genes whose deletion conferred enhanced sensitivity to Pn-AMP1. This assay identified 44 deletion strains with fitness defects in the presence of Pn-AMP1. Strong fitness defects were observed in strains with deletions of genes encoding components of several pathways and complex known to participate in the adaptive response to alkaline pH stress, including the cell wall integrity (CWI), calcineurin/Crz1, Rim101, SNF1 pathways and endosomal sorting complex required for transport (ESCRT complex). Gene ontology (GO) enrichment analysis of these genes revealed that the most highly overrepresented GO term was “cellular response to alkaline pH”. We found that 32 of the 44 deletion strains tested (72 %) showed significant growth defects compared with their wild type at alkaline pH. Furthermore, 9 deletion strains (20 %) exhibited enhanced sensitivity to Pn-AMP1 at ambient pH compared to acidic pH. Although several hundred plant AMPs have been reported, their modes of action remain largely uncharacterized. This study demonstrates that the signaling pathways that coordinate the adaptive response to alkaline pH also confer resistance to a hevein-type plant AMP in S. cerevisiae. Our findings have broad implications for the design of novel and potent antifungal agents.




Similar content being viewed by others
Abbreviations
- AMP:
-
Antimicrobial peptide
- GO:
-
Gene ontology
- CWI:
-
Cell wall integrity
- ESCRT:
-
Endosomal sorting complex required for transport
- MIC:
-
Minimum inhibitory concentration
References
Arias P, Díez-Muñiz S, García R, Nombela C, Rodríguez-Peña JM, Arroyo J (2011) Genome-wide survey of yeast mutations leading to activation of the yeast cell integrity MAPK pathway: novel insights into diverse MAPK outcomes. BMC Genom 12:390
Ariño J, Ramos J, Sychrová H (2010) Alkali metal cation transport and homeostasis in yeasts. Microbiol Mol Biol Rev 74:95–120
Broekaert WF, Cammue BPA, De Bolle MFC, Thevissen K, De Samblanx GV, Osborn RW (1997) Antimicrobial peptides from plants. Crit Rev Plant Sci 16:297–323
Casado C, González A, Platara M, Ruiz A, Ariño J (2011) The role of the protein kinase A pathway in the response to alkaline pH stress in yeast. Biochem J 438:523–533
Casamayor A, Serrano R, Platara M, Casado C, Ruiz A, Ariño J (2012) The role of the Snf1 kinase in the adaptive response of Saccharomyces cerevisiae to alkaline pH stress. Biochem J 444:39–49
Castrejon F, Gomez A, Sanz M, Duran A, Roncero C (2006) The RIM101 pathway contributes to yeast cell wall assembly and its function becomes essential in the absence of mitogen-activated protein kinase Slt2p. Eukaryot Cell 5:507–517
Cederlund A, Gudmundsson GH, Agerberth B (2011) Antimicrobial peptides important in innate immunity. FEBS J 278:3942–3951
Ericson E, Hoon S, St Onge RP, Giaever G, Nislow C (2010) Exploring gene function and drug action using chemogenomic dosage assays. Methods Enzymol 470:233–255
Galindo A, Calcagno-Pizarelli AM, Arst HN, Penalva MA (2012) An ordered pathway for the assembly of fungal ESCRT-containing ambient pH signalling complexes at the plasma membrane. J Cell Sci 125:1784–1795
García R, Bermejo C, Grau C, Pérez R, Rodríguez-Peña JM, Francois J, Nombela C, Arroyo J (2004) The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J Biol Chem 279:15183–15195
García-Olmedo F, Molina A, Alamillo JM, Rodríguez-Palenzuéla P (1998) Plant defense peptides. Biopolymers 47:479–491
Giaever G, Chu AM, Ni L, Connelly C et al (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387–391
Gustin MC, Martinac B, Saimi Y, Culbertson MR, Kung C (1986) Ion channels in yeast. Science 233:1195–1197
Hammami R, Hamida JB, Vergoten G, Fliss I (2009) PhytAMP: a database dedicated to antimicrobial plant peptides. Nucleic Acids Res 37:D963–D968
Haro R, Garciadeblas B, Rodríguez-Navarro A (1991) A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett 291:189–191
Hegedus N, Marx F (2013) Antifungal proteins: more than antimicrobials? Fungal Biol Rev 26:132–145
Jaime MD, Lopez-Llorca LV, Conesa A, Lee AY, Proctor M, Heisler LE, Gebbia M, Giaever G, Westwood JT, Nislow C (2012) Identification of yeast genes that confer resistance to chitosan oligosaccharide (COS) using chemogenomics. BMC Genom 13:267
Kinclova-Zimmermannova O, Gaskova D, Sychrova H (2006) The Na+, K+/H+-antiporter Nha1 influences the plasma membrane potential of Saccharomyces cerevisiae. FEMS Yeast Res 6:792–800
Kohno H, Tanaka K, Mino A, Umikawa M, Imamura H, Fujiwara T, Fujita Y, Hotta K, Qadota H, Watanabe T, Ohya Y, Takai Y (1996) Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J 15:6060–6068
Koo JC, Lee SY, Chun HJ, Cheong YH, Choi JS, Kawabata S, Miyagi M, Tsunasawa S, Ha KS, Bae DW, Han CD, Lee BL, Cho MJ (1998) Two hevein homologs isolated from the seed of Pharbitis nil L. exhibit potent antifungal activity. Biochim Biophys Acta 1382:80–90
Koo JC, Lee B, Young ME, Koo SC, Cooper JA, Baek D, Lim CO, Lee SY, Yun DJ, Cho MJ (2004) Pn-AMP1, a plant defense protein, induces actin depolarization in yeasts. Plant Cell Physiol 45:1669–1680
Lacerda AF, Vasconcelos EA, Pelegrini PB, Grossi de Sa MF (2014) Antifungal defensins and their role in plant defense. Front Microbiol 5:116
Lamb TM, Xu W, Diamond A, Mitchell AP (2001) Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J Biol Chem 276:1850–1856
Levin DE (2011) Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189:1145–1175
Lis M, Fuss JR, Bobek LA (2009) Exploring the mode of action of antimicrobial peptide MUC7 12-mer by fitness profiling of Saccharomyces cerevisiae genomewide mutant collection. Antimicrob Agents Chemother 53:3762–3769
Liu HP, Bretscher A (1989) Disruption of the single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell 57:233–242
López-García B, Segundo B, Coca M (2012) Antimicrobial peptides as a promising alternative for plant disease protection. In: Rajasekaran K, Cary JW, Jaynes J, Montesinos E (eds) Small wonders: peptides for disease control. ACS Symposium Series 1095:263–294
Peñalva MA, Lucena-Agell D, Arst HN (2014) Liaison alcaline: pals entice non-endosomal ESCRTs to the plasma membrane for pH signaling. Curr Opin Microbiol 22:49–59
Pierce SE, Davis RW, Nislow C, Giaever G (2007) Genome-wide analysis of barcoded Saccharomyces cerevisiae gene-deletion mutants in pooled cultures. Nat Protoc 2:2958–2974
Prior C, Potier S, Souciet JL, Sychrova H (1996) Characterization of the NHA1 gene encoding Na+/H+-antiporter of the yeast Saccharomyces cerevisiae. FEBS Lett 387:89–93
Ramos J, Alijo R, Haro R, Rodriguez-Navarro A (1994) TRK2 is not a low-affinity potassium transporter in Saccharomyces cerevisiae. J Bacteriol 176:249–252
Rodríguez-Navarro A (2000) Potassium transport in fungi and plants. Biochim Biophys Acta 1469:1–30
Ruiz A, Arino J (2007) Function and regulation of the Saccharomyces cerevisiae ENA sodium ATPase system. Eukaryot Cell 6:2175–2183
Ruiz A, Serrano R, Arino J (2008) Direct regulation of genes involved in glucose utilization by the calcium/calcineurin pathway. J Biol Chem 283:13923–13933
Serra-Cardona A, Canadell D, Ariño J (2015) Coordinate responses to alkaline pH stress in budding yeast. Microbial Cell 2:182–196
Serrano R, Ruiz A, Bernal D, Chambers JR, Ariño J (2002) The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol Microbiol 46:1319–1333
Serrano R, Bernal D, Simon E, Ariño J (2004) Copper and iron are the limiting factors for growth of the yeast Saccharomyces cerevisiae in an alkaline environment. J Biol Chem 279:19698–19704
Serrano R, Martín H, Casamayor A, Ariño J (2006) Signaling alkaline pH stress in the yeast Saccharomyces cerevisiae through the Wsc1 cell surface sensor and the Slt2 MAPK pathway. J Biol Chem 281:39785–39795
Terras FR, Eggermont K, Kovaleva V, Raikhel NV, Osborn RW, Kester A, Rees SB, Torrekens S, van Leuven F, Vanderleyden J (1995) Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell 7:573–588
Thevissen K, Terras FR, Broekaert WF (1999) Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl Environ Microbiol 65:5451–5458
Thevissen K, Ferket KK, François IE, Cammue BP (2003) Interactions of antifungal plant defensins with fungal membrane components. Peptides 24:1705–1712
Thomma BP, Cammue BP, Thevissen K (2003) Mode of action of plant defensins suggests therapeutic potential. Curr Drug Targets Infect Disord 3:1–8
van der Weerden NL, Hancock RE, Anderson MA (2010) Permeabilization of fungal hyphae by the plant defensin NaD1 occurs through a cell wall-dependent process. J Biol Chem 285:37513–37520
Viladevall L, Serrano R, Ruiz A, Domenech G, Giraldo J, Barceló A, Ariño J (2004) Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J Biol Chem 279:43614–43624
Vriens K, Cammue BPA, Thevissen K (2014) Antifungal plant defensins: mechanisms of action and production. Molecules 19:12280–12303
Acknowledgments
This project was supported by a grant from the National Institute of Agricultural Sciences (PJ01086903), RDA, Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2016_2579_MOESM1_ESM.jpg
Fig. S1 No significant changes in Pn-AMP1 sensitivity or resistance in mutant strains carrying deletion of single or multiple potassium transporters. Exponentially growing cells including wild type (WT), Δtrk1, Δtok1, Δena1, and Δnha1 were diluted and grown in 0.5X YPD broth buffered to pH 5.8, 7.5 and 8.3 in the presence or absence of Pn-AMP1. The Δslt2 was included as a control strain sensitive to Pn-AMP1. After 24 h of incubation at 30 °C, growth curves were obtained by measuring the optical density. a The MIC50 values of Pn-AMP1 were calculated from the dose–response growth curves. Error bars represent the standard error of the mean of three replicates. b Representative microplate images of growth inhibition assay. The turbid and clear wells indicate fungal growth and lack thereof, respectively (JPEG 346 kb)
425_2016_2579_MOESM2_ESM.jpg
Fig. S2 Effect of alkaline pH on Pn-AMP1 sensitivity of phytopathogenic fungi. Phytopathogenic fungi including Botritis cinerea, Fusarium fujikuroi, Penicillium digitatum and Rhizoctonia solani were obtained from the Korean Agricultural Culture Collection (KACC, South Korea) and used to isolated fungal spores as described previously (Koo et al. 1998). Potato dextrose broth (PDB) medium was buffered with Mes and Tris to a final concentration of 50 mM as described in Materials and methods. An inoculum of 104 fungal spores was diluted in 100 μl of 0.5X PDB medium at pH 5.8, 7.5 or 8.3, supplemented with various concentrations of Pn-AMP1 or water, in a 96-well microplate. Growth was measured at 600 nm after incubation for 2 days at 26 °C. The MIC50 of Pn-AMP1 was determined from the growth response curve. a The MIC50 values of Pn-AMP1 were calculated from the dose–response growth curves. Error bars represent the standard error of the mean of three replicates. b Representative microplate images of the growth inhibition assay. The turbid and clear wells indicate fungal growth and lack thereof, respectively (JPEG 757 kb)
Rights and permissions
About this article
Cite this article
Kwon, Y., Chiang, J., Tran, G. et al. Signaling pathways coordinating the alkaline pH response confer resistance to the hevein-type plant antimicrobial peptide Pn-AMP1 in Saccharomyces cerevisiae . Planta 244, 1229–1240 (2016). https://doi.org/10.1007/s00425-016-2579-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2579-2