Abstract
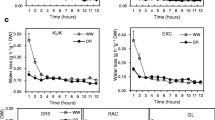
The developing leaf three of barley provides an excellent model system for the direct determination of relationships between amounts of waxes and cutin and cuticular permeance. Permeance of the cuticle was assessed via the time-course of uptake of either toluidine blue or 14C-labelled benzoic acid ([14C] BA) along the length of the developing leaf. Toluidine blue uptake only occurred within the region 0–25 mm from the point of leaf insertion (POLI). Resistance—the inverse of permeance—to uptake of [14C] BA was determined for four leaf regions and was lowest in the region 10–20 mm above POLI. At 20–30 and 50–60 mm above POLI, it increased by factors of 6 and a further 32, respectively. Above the point of emergence of leaf three from the sheath of leaf two, which was 76–80 mm above POLI, resistance was as high as at 50–60 mm above POLI. GC-FID/MS analyses of wax and cutin showed that: (1) the initial seven fold increase in cuticular resistance coincided with increase in cutin coverage and appearance of waxes; (2) the second, larger and final increase in cuticle resistance was accompanied by an increase in wax coverage, whereas cutin coverage remained unchanged; (3) cutin deposition in barley leaf epidermis occurred in parallel with cell elongation, whereas deposition of significant amounts of wax commenced as cells ceased to elongate.





Similar content being viewed by others
Abbreviations
- BA:
-
Benzoic acid
- FID:
-
Flame ionization detector
- GC:
-
Gas chromatography
- MS:
-
Mass spectrometry
- PAR:
-
Photosynthetically active radiation
- POE:
-
Point of emergence
- POLI:
-
Point of leaf insertion
- RH:
-
Relative humidity
- SEM:
-
Scanning electron microscopy
References
Barnes J, Percy K, Paul N, Jones P, McLauchlin C, Mullineaux P, Creissen G, Wellburn A (1996) The influence of UV-B radiation on the physiochemical nature of tobacco (Nicotiana tabacum L.) leaf surface. J Exp Bot 47:99–109
Barthlott W, Neinhuis C (1997) Purity of the sacred lotus, or escape from the contamination in biological science. Planta 202:1–8
Eigenbrode SD (1996) Plant surface waxes and insect behaviour. In: Kerstiens G (ed) Plant cuticles—an integrated functional approach. BIOS Scientific Publishers Limited, Oxford, pp 201–222
Espelie KE, Dean BB, Kolattukudy PE (1979) Composition of lipid-derived polymers from different anatomical regions of several plant species. Plant Physiol 64:1089–1093
Franke R, Briesen I, Wojciechowski T, Faust A, Yephremov A, Nawrath C, Schreiber L (2005) Apoplastic polyesters in Arabidopsis surface tissues—a typical suberin and a particular cutin. Phytochemistry 66:2643–2658
Fricke W (2002) Biophysical limitation of cell elongation in cereal leaves. Ann Bot 90:157–167
Fricke W, Akhiyarova G, Veselov D, Kudoyarova G (2004) Rapid and tissue-specific changes in ABA and in growth rate in response to salinity in barley leaves. J Exp Bot 55:1115–1123
Jeffree CE (1996) Structure and ontogeny of plant cuticles. In: Kerstiens G (ed) Plant cuticles—an integrated functional approach. BIOS Scientific Publishers Limited, Oxford, pp 33–82
Jenks MA, Ashworth EN (1999) Plant epicuticular waxes: function production and genetics. Hortic Rev 23:1–68
Jenks MA, Joly RJ, Peters PJ, Rich PJ, Axtell JD, Ashworth EN (1994) Chemically induced cuticle mutation affecting epidermal conductance to water vapor and disease susceptibility in Sorghum bicolor (L.) Moench. Plant Physiol 105:1239–1245
Jetter R, Schäffer S, Riederer (2000) Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant Cell Environ 23:619–628
Kerstiens G (1996a) Cuticular water permeability and its physiological significance. J Exp Bot 47:1813–1832
Kerstiens G (1996b) Signaling across the divide: a wider perspective of cuticular structure-function relationships. Trends Plant Sci 1:125–129
Kerstiens G (2006) Water transport in plant cuticles: an update. J Exp Bot 57:2493–2499
Kirsch T, Kaffarnik F, Riederer M, Schreiber L (1997) Cuticular permeability of the three tree species Prunus laurocerasus L., Ginko biloba L. and Juglans regia L.: comparative investigation of the transport properties of intact leaves, isolated cuticles and reconstituted cuticular waxes. J Exp Bot 48:1035–1045
Kunst L, Samuels AL (2003) Biosynthesis and secretion of plant cuticular wax. Prog Lipid Res 42:51–80
Lendzian KJ, Schönherr (1983) In vivo study of cutin synthesis in leaves of Clivia minitia Reg. Planta 158:70–75
Lequeu J, Fauconnier M-L, Chammaï A, Bronner R, Blée E (2003) Formation of plant cuticle: evidence for the occurrence of the peroxygenase pathway. Plant J 36:155–164
Marinari C, Wolters-Arts M (2000) Complex waxes. Plant Cell 12:1795–1798
Merida T, Schönherr J, Schmidt HW (1981) Fine structure of plant cuticles in relation to water permeability: the fine structure of the cuticle of Clivia miniata Reg. Leaves. Planta 152:259–267
Niederl S, Kirsch T, Riederer M, Schreiber L (1998) Co-permeability of 3H-labeled water and 14C-labeled organic acids across isolated plant cuticles: investigating cuticular paths of diffusion and predicting cuticular transpiration. Plant Physiol 116:117–123
Rhee Y, Hlousek-Radojcic A, Ponsamuel J, Liu D, Post-Beitenmiller D (1998) Epicuticular wax accumulation and fatty acid elongation activities are induced during leaf development in leeks. Plant Physiol 116:901–911
Richardson A, Franke R, Kerstiens G, Jarvis M, Schreiber L, Fricke W (2005) Cuticular wax deposition in growing barley (Hordeum vulgare) leaves commences in relation to the point of emergence of epidermal cells from the sheaths of older leaves. Planta 222:472–483
Riederer M, Schönherr J (1988) Development of plant cuticles–fine structure and cutin composition of Clivia miniata Reg. Leaves. Planta 174:127–138
Riederer M, Schreiber L (2001) Protecting against water loss: analysis of the barrier properties of plant cuticles. J Exp Bot 52:2023–2032
Ristic Z, Jenks MA (2002) Leaf cuticle and water loss in maize lines differing in dehydration avoidance. J Plant Physiol 159:654–651
Šantrůček J, Šimáňová E, Karbulková Šimková M, Schreiber L (2004) A new technique for measurement of water permeability of stomatous cuticular membranes isolated from Hedera helix leaves. J Exp Bot 55:1411–1422
Schmidt HW, Schönherr (1982) Development of plant cuticles–occurrence and role of non-ester bonds in cutin of Clivia miniata Reg. Leaves. Planta 156:380–384
Schreiber L, Schönherr J (1992) Analysis of foliar uptake of pesticides in barley leaves: role of epicuticular waxes and compartmentation. Pestic Sci 36:213–221
Schreiber L, Schönherr J (1993) Mobilities of organic compounds in reconstituted cuticular wax of barley leaves: determination of diffusion coefficients. Pestic Sci 38:353–361
Suh MC, Samuels AL, Jetter R, Kunst L, Pollard M, Ohlrogge J, Beisson F (2005) Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiol 139:1649–1665
Tanaka T, Tanaka H, Machida C, Watanabe M, Machida Y (2004) A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J 37:139–146
Vogg G, Fischer S, Leide J, Emmanuel E, Jetter R, Levy AA, Riederer M (2004) Tomato fruit cuticular waxes and the effects on transpiration barrier properties: functional characterisation of a mutant deficient in a very-long-chain fatty acid β-ketoacyl-CoA synthase. J Exp Bot 55:1401–1410
Acknowledgments
This research was supported by the UK Biotechnology and Biological Sciences Research Council (BBSRC), Grant 61/P18283 (to W.F.) and a studentship of the University of Paisley.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Richardson, A., Wojciechowski, T., Franke, R. et al. Cuticular permeance in relation to wax and cutin development along the growing barley (Hordeum vulgare) leaf. Planta 225, 1471–1481 (2007). https://doi.org/10.1007/s00425-006-0456-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0456-0