Abstract
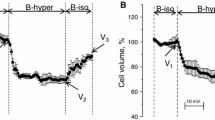
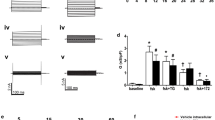
Maintenance of cell volume is a fundamental housekeeping function in eukaryotic cells. Acute cell swelling activates a regulatory volume decrease (RVD) process with poorly defined volume sensing and intermediate signaling mechanisms. Here, we analyzed the putative role of Ca2+ signaling in RVD in single substrate-adherent human lung epithelial A549 cells. Acute cell swelling was induced by perfusion of the flow-through imaging chamber with 50 % hypotonic solution at a defined fluid turnover rate. Changes in cytosolic Ca2+ concentration ([Ca2+]i) and cell volume were monitored simultaneously with ratiometric Fura-2 fluorescence and 3D reconstruction of stereoscopic single-cell images, respectively. Hypotonic challenge caused a progressive swelling peaking at ∼20 min and followed, during the next 20 min, by RVD of 60 ± 7 % of the peak volume increase. However, at the rate of swelling used in our experiments, these processes were not accompanied by a measurable increment of [Ca2+]i. Loading with intracellular Ca2+ chelator BAPTA slightly delayed peak of swelling but did not prevent RVD in 82 % of cells. Further, electrophysiology whole-cell patch-clamp experiments showed that BAPTA did not block activation of volume-regulated anion channel (VRAC) measured as swelling-induced outwardly rectifying 5-nitro-2-(3-phenylpropyl-amino) benzoic acid sensitive current. Together, our data suggest that intracellular Ca2+-mediated signaling is not essential for VRAC activation and subsequent volume restoration in A549 cells.






Similar content being viewed by others
References
Abascal F, Zardoya R (2012) LRRC8 proteins share a common ancestor with pannexins, and may form hexameric channels involved in cell-cell communication. BioEssays 34:551–560
Akita T, Fedorovich SV, Okada Y (2011) Ca2+ nanodomain-mediated component of swelling-induced volume-sensitive outwardly rectifying anion current triggered by autocrine action of ATP in mouse astrocytes. Cell Physiol Biochem 28:1181–1190
Akita T, Okada Y (2011) Regulation of bradykinin-induced activation of volume-sensitive outwardly rectifying anion channels by Ca2+ nanodomains in mouse astrocytes. J Physiol 589:3909–3927
Altamirano J, Brodwick MS, Alvarez-Leefmans FJ (1998) Regulatory volume decrease and intracellular Ca2+ in murine neuroblastoma cells studied with fluorescent probes. J Gen Physiol 112:145–160
Basavappa S, Chartouni V, Kirk K, Prpic V, Ellory JC, Mangel AW (1995) Swelling-induced chloride currents in neuroblastoma cells are calcium dependent. J Neurosci 15:3662–3666
Beckel JM, Argall AJ, Lim JC, Xia J, Lu W, Coffey EE, Macarak EJ, Shahidullah M, Delamere NA, Zode GS, Sheffield VC et al (2014) Mechanosensitive release of adenosine 5'-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain. Glia 62:1486–1501
Bibby KJ, McCulloch CA (1994) Regulation of cell volume and [Ca2+]i in attached human fibroblasts responding to anisosmotic buffers. Am J Phys 266:C1639–C1649
Boudreault F, Grygorczyk R (2004) Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol 561:499–513
Boudreault F, Grygorczyk R (2004) Evaluation of rapid volume changes of substrate-adherent cells by conventional microscopy 3D imaging. J Microsc 215:302–312
Chen B, Nicol G, Cho WK (2007) Role of calcium in volume-activated chloride currents in a mouse cholangiocyte cell line. J Membr Biol 215:1–13
Civan MM, Coca-Prados M, Peterson-Yantorno K (1994) Pathways signaling the regulatory volume decrease of cultured nonpigmented ciliary epithelial cells. Invest Ophthalmol Vis Sci 35:2876–2886
Dargan SL, Parker I (2003) Buffer kinetics shape the spatiotemporal patterns of IP3-evoked Ca2+ signals. J Physiol 553:775–788
Di CG, Ferrara MA, Miccio L, Merola F, Memmolo P, Ferraro P, Coppola G (2015) Holographic imaging of unlabelled sperm cells for semen analysis: a review. J Biophotonics 8:779–789
Doroshenko P, Neher E (1992) Volume-sensitive chloride conductance in bovine chromaffin cell membrane. J Physiol 449:197–218
Farinas J, Verkman AS (1996) Cell volume and plasma membrane osmotic water permeability in epithelial cell layers measured by interferometry. Biophys J 71:3511–3522
Gong W, Xu H, Shimizu T, Morishima S, Tanabe S, Tachibe T, Uchida S, Sasaki S, Okada Y (2004) ClC-3-independent, PKC-dependent activity of volume-sensitive Cl channel in mouse ventricular cardiomyocytes. Cell Physiol Biochem 14:213–224
Groulx N, Boudreault F, Orlov SN, Grygorczyk R (2006) Membrane reserves and hypotonic cell swelling. J Membr Biol 214:43–56
Grygorczyk R, Boudreault F, Platonova A, Orlov SN (2015) Salt and osmosensing: role of cytoplasmic hydrogel. Pflugers Arch 467:475–487
Guilak F, Erickson GR, Ting-Beall HP (2002) The effects of osmotic stress on the viscoelastic and physical properties of articular chondrocytes. Biophys J 82:720–727
Hermoso M, Olivero P, Torres R, Riveros A, Quest AF, Stutzin A (2004) Cell volume regulation in response to hypotonicity is impaired in HeLa cells expressing a protein kinase C alpha mutant lacking kinase activity. J Biol Chem 279:17681–17689
Hoffmann EK, Lambert IH, Pedersen SF (2009) Physiology of cell volume regulation in vertebrates. Physiol Rev 89:193–277
Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E (2015) PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res 43:D512–D520
Jorgensen NK, Christensen S, Harbak H, Brown AM, Lambert IH, Hoffmann EK, Simonsen LO (1997) On the role of calcium in the regulatory volume decrease (RVD) response in Ehrlich mouse ascites tumor cells. J Membr Biol 157:281–299
Kirk J, Kirk K (1994) Inhibition of volume-activated I- and taurine efflux from HeLa cells by P-glycoprotein blockers correlates with calmodulin inhibition. J Biol Chem 269:29389–29394
Kunzelmann K (2015) TMEM16, LRRC8A, bestrophin: chloride channels controlled by Ca(2+) and cell volume. Trends Biochem Sci 40:535–543
Liu G, Liu G, Chen H, Borst O, Gawaz M, Vortkamp A, Schreiber R, Kunzelmann K, Lang F (2015) Involvement of Ca2+ activated Cl- Channel Ano6 in platelet activation and apoptosis. Cell Physiol Biochem 37:1934–1944
Liu W, Xie Y, Ma J, Luo X, Nie P, Zuo Z, Lahrmann U, Zhao Q, Zheng Y, Zhao Y, Xue Y et al (2015) IBS: an illustrator for the presentation and visualization of biological sequences. Bioinformatics 31:3359–3361
McCarty NA, O'Neil RG (1992) Calcium signaling in cell volume regulation. Physiol Rev 72:1037–1061
Mola MG, Sparaneo A, Gargano CD, Spray DC, Svelto M, Frigeri A, Scemes E, Nicchia GP (2016) The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: a different point of view on the role of aquaporins. Glia 64:139–154
Mongin AA (2016) Volume-regulated anion channel—a frenemy within the brain. Pflugers Arch 468:421–441
Mongin AA, Kimelberg HK (2002) ATP potently modulates anion channel-mediated excitatory amino acid release from cultured astrocytes. Am J Physiol Cell Physiol 283:C569–C578
Mongin AA, Kimelberg HK (2005) ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am J Physiol Cell Physiol 288:C204–C213
Morley SM, Dundas SR, James JL, Gupta T, Brown RA, Sexton CJ, Navsaria HA, Leigh IM, Lane EB (1995) Temperature sensitivity of the keratin cytoskeleton and delayed spreading of keratinocyte lines derived from EBS patients. J Cell Sci 108(Pt 11):3463–3471
O'Connor ER, Kimelberg HK (1993) Role of calcium in astrocyte volume regulation and in the release of ions and amino acids. J Neurosci 13:2638–2650
Pasantes-Morales H, Morales MS (2000) Influence of calcium on regulatory volume decrease: role of potassium channels. Nephron 86:414–427
Pedersen SF, Okada Y, Nilius B (2016) Biophysics and physiology of the volume-regulated Anion Channel (VRAC)/volume-sensitive outwardly rectifying anion channel (VSOR). Pflugers Arch 468:371–383
Pedersen SF, Prenen J, Droogmans G, Hoffmann EK, Nilius B (1998) Separate swelling- and Ca2+-activated anion currents in Ehrlich ascites tumor cells. J Membr Biol 163:97–110
Platonova A, Boudreault F, Kapilevich LV, Maksimov GV, Ponomarchuk O, Grygorczyk R, Orlov SN (2014) Temperature-induced inactivation of cytoplasmic biogel osmosensing properties is associated with suppression of regulatory volume decrease in A549 cells. J Membr Biol 247:571–579
Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A (2014) SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157:447–458
Quiros-Gonzalez I, Sainz RM, Hevia D, Mayo JC (2011) MnSOD drives neuroendocrine differentiation, androgen independence, and cell survival in prostate cancer cells. Free Radic Biol Med 50:525–536
Rudkouskaya A, Chernoguz A, Haskew-Layton RE, Mongin AA (2008) Two conventional protein kinase C isoforms, alpha and beta I, are involved in the ATP-induced activation of volume-regulated anion channel and glutamate release in cultured astrocytes. J Neurochem 105:2260–2270
Shen MR, Chou CY, Browning JA, Wilkins RJ, Ellory JC (2001) Human cervical cancer cells use Ca2+ signalling, protein tyrosine phosphorylation and MAP kinase in regulatory volume decrease. J Physiol 537:347–362
Shen MR, Furla P, Chou CY, Ellory JC (2002) Myosin light chain kinase modulates hypotonicity-induced Ca2+ entry and Cl- channel activity in human cervical cancer cells. Pflugers Arch 444:276–285
Sirianant L, Wanitchakool P, Ousingsawat J, Benedetto R, Zormpa A, Cabrita I, Schreiber R, Kunzelmann K (2016) Non-essential contribution of LRRC8A to volume regulation. Pflugers Arch 468:805–816
Sit ST, Manser E (2011) Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci 124:679–683
Souza MM, Boyle RT (2001) A moderate decrease in temperature inhibits the calcium signaling mechanism(s) of the regulatory volume decrease in chick embryo cardiomyocytes. Braz J Med Biol Res 34:137–141
Speake T, Douglas IJ, Brown PD (1998) The role of calcium in the volume regulation of rat lacrimal acinar cells. J Membr Biol 164:283–291
Szucs G, Heinke S, Droogmans G, Nilius B (1996) Activation of the volume-sensitive chloride current in vascular endothelial cells requires a permissive intracellular Ca2+ concentration. Pflugers Arch 431:467–469
Tatur S, Groulx N, Orlov SN, Grygorczyk R (2007) Ca2 + −dependent ATP release from A549 cells involves synergistic autocrine stimulation by co-released uridine nucleotides. J Physiol 584:419–435
Thul R, Coombes S, Roderick HL, Bootman MD (2012) Subcellular calcium dynamics in a whole-cell model of an atrial myocyte. Proc Natl Acad Sci U S A 109:2150–2155
Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ (2014) Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344:634–638
Yap KL, Kim J, Truong K, Sherman M, Yuan T, Ikura M (2000) Calmodulin target database. J Struct Funct Genom 1:8–14
Zhou HX, Rivas G, Minton AP (2008) Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu Rev Biophys 37:375–397
Acknowledgments
This study was supported by Natural Science and Engineering Research Council of Canada discovery grant RGPIN 435517-2013 (RG).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ponomarchuk, O., Boudreault, F., Orlov, S.N. et al. Calcium is not required for triggering volume restoration in hypotonically challenged A549 epithelial cells. Pflugers Arch - Eur J Physiol 468, 2075–2085 (2016). https://doi.org/10.1007/s00424-016-1896-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-016-1896-4