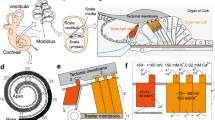
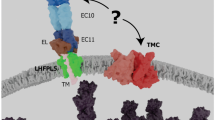
Abstract
Identification of the auditory hair cell mechano-electrical transduction (hcMET) channel has been a major focus in the hearing research field since the 1980s when direct mechanical gating of a transduction channel was proposed (Corey and Hudspeth J Neurosci 3:962–976, 1983). To this day, the molecular identity of this channel remains controversial. However, many of the hcMET channel’s properties have been characterized, including pore properties, calcium-dependent ion permeability, rectification, and single channel conductance. At this point, elucidating the molecular identity of the hcMET channel will provide new tools for understanding the mechanotransduction process. This review discusses the significance of identifying the hcMET channel, the difficulties associated with that task, as well as the establishment of clear criteria for this identification. Finally, we discuss potential candidate channels in light of these criteria.



Similar content being viewed by others
References
Alloui A, Zimmermann K, Mamet J, Duprat F, Noël J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C, Borsotto M, Romey G, Heurteaux C, Reeh P, Eschalier A, Lazdunski M (2006) TREK-1, a K+ channel involved in polymodal pain perception. EMBO J 25(11):2368–2376
Anishkin A, Loukin SH, Teng J, Kung C (2014) Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc Natl Acad Sci U S A 111(22):7898–7905
Asai Y, Holt JR, Géléoc GSG (2010) A quantitative analysis of the spatiotemporal pattern of transient receptor potential gene expression in the developing mouse cochlea. J Assoc Res Otolaryngol 11(1):27–37
Atiba-Davies M, Noben-Trauth K (2007) TRPML3 and hearing loss in the varitint-waddler mouse. Biochim Biophys Acta 1772(8):1028–1031
Bahloul A, Michel V, Hardelin J-P, Nouaille S, Hoos S, Houdusse A, England P, Petit C (2010) Cadherin-23, myosin VIIa and harmonin, encoded by Usher syndrome type I genes, form a ternary complex and interact with membrane phospholipids. Hum Mol Genet 19(18):3557–3565
Bass RB, Strop P, Barclay M, Rees DC (2002) Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298(5598):1582–1587
Bautista DM, Jordt S-E, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124(6):1269–1282
Beurg M, Evans MG, Hackney CM, Fettiplace R (2006) A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci 26(43):10992–11000
Beurg M, Fettiplace R, Nam J-H, Ricci AJ (2009) Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci 12(5):553–558
Beurg M, Kim KX, Fettiplace R (2014) Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants. J Gen Physiol 144(1):55–69
Beurg M, Nam J-H, Chen Q, Fettiplace R (2010) Calcium balance and mechanotransduction in rat cochlear hair cells. J Neurophysiol 104(1):18–34
Beurg M, Nam J-H, Crawford A, Fettiplace R (2008) The actions of calcium on hair bundle mechanics in mammalian cochlear hair cells. Biophys J 94(7):2639–2653
Bianchi L (2007) Mechanotransduction: touch and feel at the molecular level as modeled in Caenorhabditis elegans. Mol Neurobiol 36(3):254–271
Biel M, Zong X, Ludwig A, Sautter A, Hofmann F (1999) Structure and function of cyclic nucleotide-gated channels. Rev Physiol Biochem Pharmacol 135:151–171
Blount P, Sukharev S, Kung C (1997) A mechanosensitive channel protein and its gene in E. coli. Gravit Space Biol Bull 10(2):43–47
Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R (2006) A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci 26(18):4835–4840
Brohawn SG, Su Z, MacKinnon R (2014) Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc Natl Acad Sci U S A 111(9):3614–3619
Brown AL, Liao Z, Goodman MB (2008) MEC-2 and MEC-6 in the Caenorhabditis elegans sensory mechanotransduction complex: auxiliary subunits that enable channel activity. J Gen Physiol 131(6):605–616
Catterall WA (2011) Voltage-gated calcium channels. Cold Spring Harb Perspect Biol 3(8):a003947
Chatzigeorgiou M, Bang S, Hwang SW, Schafer WR (2013) tmc-1 encodes a sodium-sensitive channel required for salt chemosensation in C. elegans. Nature 494(7435):95–99
Corey DP, García-Añoveros J, Holt JR, Kwan KY, Lin S-Y, Vollrath MA, Amalfitano A, Cheung EL-M, Derfler BH, Duggan A, Géléoc GSG, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang D-S (2004) TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432(7018):723–730
Corey DP, Hudspeth AJ (1979) Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281(5733):675–677
Corey DP, Hudspeth AJ (1983) Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci 3(5):962–976
Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A (2010) Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330(6000):55–60
Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A (2012) Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483(7388):176–181
Denk W, Holt JR, Shepherd GM, Corey DP (1995) Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron 15(6):1311–1321
Dzeja C, Hagen V, Kaupp UB, Frings S (1999) Ca2+ permeation in cyclic nucleotide-gated channels. EMBO J 18(1):131–144
Eastwood AL, Goodman MB (2012) Insight into DEG/ENaC channel gating from genetics and structure. Physiology (Bethesda) 27(5):282–290
Effertz T, Nadrowski B, Piepenbrock D, Albert JT, Göpfert MC (2012) Direct gating and mechanical integrity of Drosophila auditory transducers require TRPN1. Nat Neurosci 15(9):1198–1200
Effertz T, Wiek R, Göpfert MC (2011) NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol 21(21):592–597
Farris HE, LeBlanc CL, Goswami J, Ricci AJ (2004) Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J Physiol 558(Pt 3):769–792
Fesenko EE, Kolesnikov SS, Lyubarsky AL (1985) Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313(6000):310–313
Fettiplace R (2009) Defining features of the hair cell mechanoelectrical transducer channel. Pflugers Arch 458(6):1115–1123
Frings S, Seifert R, Godde M, Kaupp UB (1995) Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron 15(1):169–179
Gerka-Stuyt J, Au A, Peachey NS, Alagramam KN (2013) Transient receptor potential melastatin 1: a hair cell transduction channel candidate. PLoS One 8(10):e77213
Gerstner A, Zong X, Hofmann F, Biel M (2000) Molecular cloning and functional characterization of a new modulatory cyclic nucleotide-gated channel subunit from mouse retina. J Neurosci 20(4):1324–1332
Gillespie D, Boda D (2008) The anomalous mole fraction effect in calcium channels: a measure of preferential selectivity. Biophys J 95(6):2658–2672
Gleason MR, Nagiel A, Jamet S, Vologodskaia M, López-Schier H, Hudspeth AJ (2009) The transmembrane inner ear (Tmie) protein is essential for normal hearing and balance in the zebrafish. Proc Natl Acad Sci U S A 106(50):21347–21352
Goodman MB, Schwarz EM (2003) Transducing touch in Caenorhabditis elegans. Annu Rev Physiol 65:429–452
Gradogna A, Babini E, Picollo A, Pusch M (2010) A regulatory calcium-binding site at the subunit interface of CLC-K kidney chloride channels. J Gen Physiol 136(3):311–323
Grati M, Kachar B (2011) Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc Natl Acad Sci U S A 108(28):11476–11481
Hackney CM, Furness DN, Benos DJ, Woodley JF, Barratt J (1992) Putative immunolocalization of the mechanoelectrical transduction channels in mammalian cochlear hair cells. Proc Biol Sci 248(1323):215–221
Haynes LW, Yau KW (1990) Single-channel measurement from the cyclic GMP-activated conductance of catfish retinal cones. J Physiol 429:451–481
Helyer R, Cacciabue-Rivolta D, Davies D, Rivolta MN, Kros CJ, Holley MC (2007) A model for mammalian cochlear hair cell differentiation in vitro: effects of retinoic acid on cytoskeletal proteins and potassium conductances. Eur J Neurosci 25(4):957–973
Hess P, Tsien RW (1984) Mechanism of ion permeation through calcium channels. Nature 309(5967):453–456
Houser DS, Finneran JJ (2006) A comparison of underwater hearing sensitivity in bottlenose dolphins (Tursiops truncatus) determined by electrophysiological and behavioral methods. J Acoust Soc Am 120(3):1713–1722
Housley GD, Greenwood D, Ashmore JF (1992) Localization of cholinergic and purinergic receptors on outer hair cells isolated from the guinea-pig cochlea. Proc Biol Sci 249(1326):265–273
Howard J, Hudspeth AJ (1987) Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog’s saccular hair cell. Proc Natl Acad Sci U S A 84(9):3064–3068
Howard J, Hudspeth AJ (1988) Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog’s saccular hair cell. Neuron 1(3):189–199
Hudspeth AJ (2005) How the ear’s works work: mechanoelectrical transduction and amplification by hair cells. C R Biol 328(2):155–162
Ishibashi T, Takumida M, Akagi N, Hirakawa K, Anniko M (2008) Expression of transient receptor potential vanilloid (TRPV) 1, 2, 3, and 4 in mouse inner ear. Acta Otolaryngol 128(12):1286–1293
Kaupp UB, Seifert R (2002) Cyclic nucleotide-gated ion channels. Physiol Rev 82(3):769–824
Kawashima Y, Géléoc GSG, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, Griffith AJ (2011) Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest 31(34):12241–12250
Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, Kachar B (2007) Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449(7158):87–91
Kellenberger S, Schild L (2002) Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82(3):735–767
Kim KX, Beurg M, Hackney CM, Furness DN, Mahendrasingam S, Fettiplace R (2013) The role of transmembrane channel-like proteins in the operation of hair cell mechanotransducer channels. J Gen Physiol 142(5):493–505
Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, Naz S, Arnaud D, Drury S, Mo J, Makishima T, Ghosh M, Menon PSN, Deshmukh D, Oddoux C, Ostrer H, Khan S, Riazuddin S, Deininger PL, Hampton LL, Sullivan SL, Battey J, James F, Keats BJB, Wilcox ER, Friedman TB, Griffith AJ (2002) Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet 30(3):277–284
Kurima K, Yang Y, Sorber K, Griffith AJ (2003) Characterization of the transmembrane channel-like (TMC) gene family: functional clues from hearing loss and epidermodysplasia verruciformis. Genomics 82(3):300–308
Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang D-S, Woolf CJ, Corey DP (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50(2):277–289
Lehnert BP, Baker AE, Gaudry Q, Chiang A-S, Wilson RI (2013) Distinct roles of TRP channels in auditory transduction and amplification in drosophila. Neuron 77(1):115–128
Levsky JM, Singer RH (2003) Fluorescence in situ hybridization: past, present and future. J Cell Sci 116(Pt 14):2833–2838
Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103(3):525–535
Lin Z, Perez P, Sun Z, Liu J-J, Shin JH, Hyrc KL, Samways D, Egan T, Holley MC, Bao J (2012) Reprogramming of single-cell-derived mesenchymal stem cells into hair cell-like cells. Otol Neurotol 33(9):1648–1655
Lisenbee CS, Karnik SK, Trelease RN (2003) Overexpression and mislocalization of a tail-anchored GFP redefines the identity of peroxisomal ER. Traffic 4(7):491–501
Liu H, Pecka JL, Zhang Q, Soukup GA, Beisel KW, He DZZ (2014) Characterization of transcriptomes of cochlear inner and outer hair cells. J Neurosci 34(33):11085–11095
Lo P-K, Lee JS, Chen H, Reisman D, Berger FG, Sukumar S (2013) Cytoplasmic mislocalization of overexpressed FOXF1 is associated with the malignancy and metastasis of colorectal adenocarcinomas. Exp Mol Pathol 94(1):262–269
Longo-Guess CM, Gagnon LH, Cook SA, Wu J, Zheng QY, Johnson KR (2005) A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc Natl Acad Sci U S A 102(22):7894–7899
Lumpkin EA, Marquis RE, Hudspeth AJ (1997) The selectivity of the hair cell’s mechanoelectrical-transduction channel promotes Ca2+ flux at low Ca2+ concentrations. Proc Natl Acad Sci U S A 94(20):10997–11002
Maeda R, Kindt KS, Mo W, Morgan CP, Erickson T, Zhao H, Clemens-Grisham R, Barr-Gillespie PG, Nicolson T (2014) Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proc Natl Acad Sci USA
Marcotti W, Corns LF, Desmonds T, Kirkwood NK, Richardson GP, Kros CJ (2014) Transduction without tip links in cochlear hair cells is mediated by ion channels with permeation properties distinct from those of the mechano-electrical transducer channel. J Neurosci 34(16):5505–5514
Nagata K, Duggan A, Kumar G, García-Añoveros J (2005) Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25(16):4052–4061
Noël J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M (2009) The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J 28(9):1308–1318
O’Hagan R, Chalfie M, Goodman MB (2005) The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci 8(1):43–50
Ohmori H (1985) Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol 359:189–217
Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S (2010) Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell 141(4):704–716
Pan B, Géléoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR (2013) TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron
Pan B, Waguespack J, Schnee ME, Leblanc C, Ricci AJ (2012) Permeation properties of the hair cell mechanotransducer channel provide insight into its molecular structure. J Neurophysiol 107(9):2408–2420
Park S, Lee J-H, Cho H-J, K-y L, Kim MO, Yun B-W, Ryoo Z (2013) tmie is required for gentamicin uptake by the hair cells of mice. Comp Med 63(2):136–142
Pedersen SF, Owsianik G, Nilius B (2005) TRP channels: an overview. Cell Calcium 38(3–4):233–252
Peng B-G, Ahmad S, Chen S, Chen P, Price MP, Lin X (2004) Acid-sensing ion channel 2 contributes a major component to acid-evoked excitatory responses in spiral ganglion neurons and plays a role in noise susceptibility of mice. J Neurosci 24(45):10167–10175
Peng AW, Salles FT, Pan B, Ricci AJ (2011) Integrating the biophysical and molecular mechanisms of auditory hair cell mechanotransduction. Nat Commun 2:523
Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B (2002) Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 418(6901):942–948
Perozo E, Rees DC (2003) Structure and mechanism in prokaryotic mechanosensitive channels. Curr Opin Struct Biol 13(4):432–442
Pickles JO, Brix J, Comis SD, Gleich O, Köppl C, Manley GA, Osborne MP (1989) The organization of tip links and stereocilia on hair cells of bird and lizard basilar papillae. Hear Res 41(1):31–41
Pickles JO, Comis SD, Osborne MP (1984) Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res 15(2):103–112
Quick K, Zhao J, Eijkelkamp N, Linley JE, Rugiero F, Cox JJ, Raouf R, Gringhuis M, Sexton JE, Abramowitz J, Taylor R, Forge A, Ashmore J, Kirkwood N, Kros CJ, Richardson GP, Freichel M, Flockerzi V, Birnbaumer L, Wood JN (2012) TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol 2(5):120068
Quintero OA, Moore JE, Unrath WC, Manor U, Salles FT, Grati M, Kachar B, Yengo CM (2010) Intermolecular autophosphorylation regulates myosin IIIa activity and localization in parallel actin bundles. J Biol Chem 285(46):35770–35782
Ramakrishnan NA, Drescher MJ, Barretto RL, Beisel KW, Hatfield JS, Drescher DG (2009) Calcium-dependent binding of HCN1 channel protein to hair cell stereociliary tip link protein protocadherin 15 CD3. J Biol Chem 284(5):3227–3238
Ramakrishnan NA, Drescher MJ, Khan KM, Hatfield JS, Drescher DG (2012) HCN1 and HCN2 proteins are expressed in cochlear hair cells: HCN1 can form a ternary complex with protocadherin 15 CD3 and F-actin-binding filamin A or can interact with HCN2. J Biol Chem 287(45):37628–37646
Ricci AJ, Crawford AC, Fettiplace R (2003) Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron 40(5):983–990
Ricci AJ, Fettiplace R (1998) Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J Physiol 506(Pt 1):159–173
Ricci AJ, Gray-Keller M, Fettiplace R (2000) Tonotopic variations of calcium signalling in turtle auditory hair cells. J Physiol 524(Pt 2):423–436
Ricci AJ, Kennedy HJ, Crawford AC, Fettiplace R (2005) The transduction channel filter in auditory hair cells. J Neurosci 25(34):7831–7839
Roza C, Puel J-L, Kress M, Baron A, Diochot S, Lazdunski M, Waldmann R (2004) Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing. J Physiol 558(Pt 2):659–669
Rüsch A, Hummler E (1999) Mechano-electrical transduction in mice lacking the alpha-subunit of the epithelial sodium channel. Hear Res 131(1–2):170–176
Saleem F, Rowe ICM, Shipston MJ (2009) Characterization of BK channel splice variants using membrane potential dyes. Br J Pharmacol 156(1):143–152
Salles FT, Merritt J, Raymond C, Manor U, Dougherty GW, Sousa AD, Moore JE, Yengo CM, Dosé AC, Kachar B (2009) Myosin IIIa boosts elongation of stereocilia by transporting espin 1 to the plus ends of actin filaments. Nat Cell Biol 11(4):443–450
Shabbir MI, Ahmed ZM, Khan SY, Riazuddin S, Waryah AM, Khan SN, Camps RD, Ghosh M, Kabra M, Belyantseva IA, Friedman TB, Riazuddin S (2006) Mutations of human TMHS cause recessively inherited non-syndromic hearing loss. J Med Genet 43(8):634–640
Shin J-B, Krey JF, Hassan A, Metlagel Z, Tauscher AN, Pagana JM, Sherman NE, Jeffery ED, Spinelli KJ, Zhao H, Wilmarth PA, Choi D, David LL, Auer M, Barr-Gillespie PG (2013) Molecular architecture of the chick vestibular hair bundle. Nat Neurosci 16(3):365–374
Shin MJ, Lee J-H, Yu DH, Kim HJ, Bae KB, Yuh HS, Kim MO, Hyun B-H, Lee S, Park R, Ryoo ZY (2010) Spatiotemporal expression of tmie in the inner ear of rats during postnatal development. Comp Med 60(4):288–294
Sukharev SI, Blount P, Martinac B, Guy HR, Kung C (1996) MscL: a mechanosensitive channel in Escherichia coli. Soc Gen Physiol Ser 51:133–141
Takumida M, Ishibashi T, Hamamoto T, Hirakawa K, Anniko M (2009) Expression of transient receptor potential channel melastin (TRPM) 1–8 and TRPA1 (ankyrin) in mouse inner ear. Acta Otolaryngol 129(10):1050–1060
Tang L, Gamal El-Din TM, Payandeh J, Martinez GQ, Heard TM, Scheuer T, Zheng N, Catterall WA (2014) Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature 505(7481):56–61
Tousson A, Alley CD, Sorscher EJ, Brinkley BR, Benos DJ (1989) Immunochemical localization of amiloride-sensitive sodium channels in sodium-transporting epithelia. J Cell Sci 93(Pt 2):349–362
van Aken AFJ, Atiba-Davies M, Marcotti W, Goodyear RJ, Bryant JE, Richardson GP, Noben-Trauth K, Kros CJ (2008) TRPML3 mutations cause impaired mechano-electrical transduction and depolarization by an inward-rectifier cation current in auditory hair cells of varitint-waddler mice. J Physiol 586(Pt 22):5403–5418
Voets T, Talavera K, Owsianik G, Nilius B (2005) Sensing with TRP channels. Nat Chem Biol 1(2):85–92
Waguespack J, Salles FT, Kachar B, Ricci AJ (2007) Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci 27(50):13890–13902
Wang Z, Jiang Y, Lu L, Huang R, Hou Q, Shi F (2007) Molecular mechanisms of cyclic nucleotide-gated ion channel gating. J Genet Genom 34(6):477–485
Wang L, Zou J, Shen Z, Song E, Yang J (2012) Whirlin interacts with espin and modulates its actin-regulatory function: an insight into the mechanism of Usher syndrome type II. Hum Mol Genet 21(3):692–710
Xie J, Price MP, Wemmie JA, Askwith CC, Welsh MJ (2003) ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J Neurophysiol 89(5):2459–2465
Xiong W, Grillet N, Elledge HM, Wagner TFJ, Zhao B, Johnson KR, Kazmierczak P, Müller U (2012) TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell 151(6):1283–1295
Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN (2013) Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493(7431):221–225
Yang J-J, Su M-C, Chien K-H, Hsin C-H, Li S-Y (2010) Identification of novel variants in the TMIE gene of patients with nonsyndromic hearing loss. Int J Pediatr Otorhinolaryngol 74(5):489–493
Yau KW, Baylor DA (1989) Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci 12:289–327
Zallocchi M, Meehan DT, Delimont D, Rutledge J, Gratton MA, Flannery J, Cosgrove D (2012) Role for a novel Usher protein complex in hair cell synaptic maturation. PLoS One 7(2):e30573
Zhang W, Yan Z, Jan LY, Jan YN (2013) Sound response mediated by the TRP channels NOMPC, NANCHUNG, and INACTIVE in chordotonal organs of Drosophila larvae. Proc Natl Acad Sci U S A 110(33):13612–13617
Acknowledgments
This work was supported by DAAD (German academic exchange service) to T.E., by the NSF-GRFP to A.L.S., and by RO1 DC003896 from NIDCD to A.J.R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Effertz, T., Scharr, A.L. & Ricci, A.J. The how and why of identifying the hair cell mechano-electrical transduction channel. Pflugers Arch - Eur J Physiol 467, 73–84 (2015). https://doi.org/10.1007/s00424-014-1606-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1606-z