Abstract
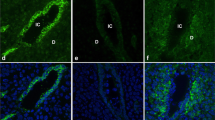
At the time of implantation, uterine luminal epithelial cells undergo a dramatic change in all plasma membrane domains. Changes in the basolateral plasma membrane at the time of implantation include progression from smooth to highly tortuous, as well as a loss of integrin-based focal adhesions. Another aspect of the basolateral plasma membrane that has not been studied in uterine epithelial cells are caveolae, which are omega-shaped invaginations of the plasma membrane known to be involved in endocytosis and contribute to membrane curvature. The current study investigated caveolin, a major protein of caveolae, to explore the possible roles that they play in the remodelling of the basolateral plasma membrane of uterine epithelial cells during early pregnancy in the rat. Morphological caveolae were found at the time of implantation and were significantly increased compared to day 1 of pregnancy. Caveolins 1 and 2 were found to shift to the basolateral plasma membrane of uterine epithelial cells at the time of implantation as well as when treated with progesterone alone, and in combination with oestrogen. A statistically significant increase in the amount of caveolin-1 and a decrease in caveolin-2 protein in uterine epithelial cells was observed at the time of implantation. Caveolin-1 also co-immunoprecipitated with integrin β1 on day 1 of pregnancy, which is a protein that has been reported to be found in integrin-based focal adhesions at the basolateral membrane on day 1 of pregnancy. The localisation and expression of caveolin-1 at the time of implantation is consistent with the presence and increase of morphological caveolae seen at this time. The localisation and expression of caveolins 1 and 2 in luminal uterine epithelium at the time of implantation suggest a role in trafficking proteins and the maintenance of a polarised epithelium.






Similar content being viewed by others
References
Anderson RG (1993) Caveolae: where incoming and outgoing messengers meet. Paper presented at the proceedings of the National Academy of Sciences, 12 Feb
Cheng ZJ, Singh RD, Marks DL, Pagano RE (2006) Membrane microdomains, caveolae, and caveolar endocytosis of sphingolipids. Mol Membr Biol 23(1):101–110. doi:10.1080/09687860500460041
del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA (2005) Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol 7(9):901–908. doi:10.1038/ncb1293
Fabbri M, Meglio S, Gagliani MC, Consonni E, Molteni R, Bender JR, Tacchetti C, Pardi R (2005) Dynamic partitioning into lipid rafts controls the endo-exocytic cycle of the αL/β2 integrin, LFA-1, during leukocyte chemotaxis. Mol Biol Cell 16(12):5793–5803
Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA (2006) Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem 281(36):26391–26399. doi:10.1074/jbc.M602577200
Hoshino Y, Shannon WA, Seligman AM (1976) Study of ferrocyanide-reduced osmium tetroxide as a stain and cytochemical agent. Acta Histochem Cytochem 9(2):125–136
Hu G, Minshall RD (2009) Regulation of transendothelial permeability by Src kinase. Microvasc Res 77(1):21–25. doi:10.1016/j.mvr.2008.10.002
Huang PL (2003) Endothelial nitric oxide synthase and endothelial dysfunction. Curr Hypertens Rep 5(6):473–480
Ikonen E (2001) Roles of lipid rafts in membrane transport. Curr Opin Cell Biol 13(4):470–477
Kaneko Y, Lindsay LA, Murphy CR (2008) Focal adhesions disassemble during early pregnancy in rat uterine epithelial cells. Reprod Fertil Dev 20(8):892–899
Kaneko Y, Lecce L, Murphy CR (2009) Ovarian hormones regulate expression of the focal adhesion proteins, talin and paxillin, in rat uterine luminal but not glandular epithelial cells. Histochem Cell Biol 132(6):613–622
Kaneko Y, Lecce L, Day ML, Murphy CR (2011) β1 and β3 integrins disassemble from basal focal adhesions and β3 integrin is later localised to the apical plasma membrane of rat uterine luminal epithelial cells at the time of implantation. Reprod Fertil Dev 23(3):481
Karnovsky MJ (1971) Use of ferrocyanide-reduced osmium tetroxide in electron microscopy. Abstracts of the American Society of Cell Biology, Eleventh Annual Meeting, New Orleans, p 146
Kirkham M, Nixon SJ, Howes MT, Abi-Rached L, Wakeham DE, Hanzal-Bayer M, Ferguson C, Hill MM, Fernandez-Rojo M, Brown DA, Hancock JF, Brodsky FM, Parton RG (2008) Evolutionary analysis and molecular dissection of caveola biogenesis. J Cell Sci 121(12):2075–2086. doi:10.1242/jcs.024588
Lecce L, Lindsay L, Kaneko Y, Murphy CR (2013) ICAM-2 and lipid rafts disappear from the basal plasma membrane of uterine epithelial cells during early pregnancy in rats. Cell Tissue Res 353(3):563–573. doi:10.1007/s00441-013-1656-0
Lee IH, Campbell CR, Song SH, Day ML, Kumar S, Cook DI, Dinudom A (2009) The activity of the epithelial sodium channels is regulated by caveolin-1 via a Nedd4-2-dependent mechanism. J Biol Chem 284(19):12663–12669. doi:10.1074/jbc.M809737200
Li S, Seitz R, Lisanti MP (1996) Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem 271(7):3863–3868
Lisanti M, Tang Z, Scherer P, Sargiacomo M (1995) Caveolae purification and glycosylphosphatidylinositol-linked protein sorting in polarized epithelia. Methods Enzymol 250:655–668
Lo WK, Zhou CJ, Reddan J (2004) Identification of caveolae and their signature proteins caveolin 1 and 2 in the lens. Exp Eye Res 79(4):487–498. doi:10.1016/j.exer.2004.06.019
Luxford KA, Murphy CR (1992) Reorganization of the apical cytoskeleton of uterine epithelial cells during early pregnancy in the rat: a study with myosin subfragment 1. Biol Cell 74(2):195–202
Matveev S, Li X, Everson W, Smart EJ (2001) The role of caveolae and caveolin in vesicle-dependent and vesicle-independent trafficking. Adv Drug Deliv Rev 49(3):237–250
Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ (2006) Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol 8(2):U113–U115. doi:10.1038/Ncb1356
Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB (2003) Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol 285(6):L1179–L1183. doi:10.1152/ajplung.00242.2003
Mora R, Bonilha VL, Marmorstein A, Scherer PE, Brown D, Lisanti MP, Rodriguez-Boulan E (1999) Caveolin-2 localizes to the golgi complex but redistributes to plasma membrane, caveolae, and rafts when co-expressed with caveolin-1. J Biol Chem 274(36):25708–25717. doi:10.1074/jbc.274.36.25708
Mora RC, Bonilha VL, Shin BC, Hu J, Cohen-Gould L, Bok D, Rodriguez-Boulan E (2006) Bipolar assembly of caveolae in retinal pigment epithelium. Am J Physiol Cell Physiol 290(3):C832–C843. doi:10.1152/ajpcell.00405.2005
Murphy CR (1995) The cytoskeleton of uterine epithelial cells: a new player in uterine receptivity and the plasma membrane transformation. Hum Reprod Update 1(6):567–580
Murphy C (2001) The plasma membrane transformation: a key concept in uterine receptivity. Reprod Med Rev 9(03):197–208
Murphy CR (2004) Uterine receptivity and the plasma membrane transformation. Cell Res 14(4):259–267. doi:10.1038/sj.cr.7290227
Murphy CR, Dwarte DM (1987) Increase in cholesterol in the apical plasma membrane of uterine epithelial cells during early pregnancy in the rat. Acta Anat (Basel) 128(1):76–79
Murphy CR, Martin B (1985) Cholesterol in the plasma membrane of uterine epithelial cells: a freeze-fracture cytochemical study with digitonin. J Cell Sci 78:163–172
Murphy C, Rogers A (1981) Effects of ovarian hormones on cell membranes in the rat uterus. III. The surface carbohydrates at the apex of the luminal epithelium. Cell Biophys 3(4):305–320
Murphy CR, Shaw TJ (1994) Plasma membrane transformation: a common response of uterine epithelial cells during the peri-implantation period. Cell Biol Int 18(12):1115–1128. doi:10.1006/cbir.1994.1038
Murphy CR, Swift JG, Mukherjee TM, Rogers AW (1981) Effects of ovarian hormones on cell membranes in the rat uterus. II. Freeze-fracture studies on tight junctions of the lateral plasma membrane of the luminal epithelium. Cell Biophys 3(1):57–69. doi:10.1007/BF02782153
Navarro A, Anand-Apte B, Parat MO (2004) A role for caveolae in cell migration. FASEB J 18(15):1801–1811. doi:10.1096/fj.04-2516rev
Orlichenko L, Huang B, Krueger E, McNiven M (2005) Epithelial growth factor-induced phosphorylation of caveolin 1 at tyrosine 14 stimulates caveolae formation in epithelial cells. J Biol Chem 281(8):4570–4579
Parolini I, Sargiacomo M, Galbiati F, Rizzo G, Grignani F, Engelman JA, Okamoto T, Ikezu T, Scherer PE, Mora R, Rodriguez-Boulan E, Peschle C, Lisanti MP (1999) Expression of caveolin-1 is required for the transport of caveolin-2 to the plasma membrane—retention of caveolin-2 at the level of the Golgi complex. J Biol Chem 274(36):25718–25725. doi:10.1074/jbc.274.36.25718
Parr MB (1980) Endocytosis at the basal and lateral membranes of rat uterine epithelial-cells during early-pregnancy. J Reprod Fertil 60(1):95–99
Parr M (1982) Apical vesicles in the rat uterine epithelium during early pregnancy: a morphometric study. Biol Reprod 26(5):915–924
Petrache HI, Harries D, Parsegian VA (2005) Alteration of lipid membrane rigidity by cholesterol and its metabolic precursors. Macromol Symp 219(1):39–50. doi:10.1002/masy.200550105
Predescu D, Horvat R, Predescu S, Palade GE (1994) transcytosis in the continuous endothelium of the myocardial microvasculature is inhibited by N-ethylmaleimide. Proc Natl Acad Sci USA 91(8):3014–3018. doi:10.1073/pnas.91.8.3014
Preston AM, Lindsay LA, Murphy CR (2004) Progesterone treatment and the progress of early pregnancy reduce desmoglein 1&2 staining along the lateral plasma membrane in rat uterine epithelial cells. Acta Histochem 106(5):345–351. doi:10.1016/j.acthis.2004.07.004
Psychoyos A (1973) Hormonal control of ovoimplantation. Vitam Horm 31:201–256
Salanueva IJ, Cerezo A, Guadamillas MC, del Pozo MA (2007) Integrin regulation of caveolin function. J Cell Mol Med 11(5):969–980. doi:10.1111/j.1582-4934.2007.00109.x
Schlegel A, Wang C, Katzenellenbogen BS, Pestell RG, Lisanti MP (1999) Caveolin-1 potentiates estrogen receptor alpha (ERalpha) signaling. Caveolin-1 drives ligand-independent nuclear translocation and activation of ERalpha. J Biol Chem 274(47):33551–33556
Sowa G (2011) Novel insights into the role of caveolin-2 in cell- and tissue-specific signaling and function. Biochem Res Int 2011:1–9
Sun Y, Hu G, Zhang X, Minshall RD (2009) Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ Res 105 (7):676–685, 615 p following 685. doi:10.1161/CIRCRESAHA.109.201673
Tabas I (2002) Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Investig 110(7):905–911. doi:10.1172/JCI16452
Turi A, Kiss AL, Mullner N (2001) Estrogen downregulates the number of caveolae and the level of caveolin in uterine smooth muscle. Cell Biol Int 25(8):785–794. doi:10.1006/cbir.2001.0769
Vogel U, Sandvig K, van Deurs B (1998) Expression of caveolin-1 and polarized formation of invaginated caveolae in Caco-2 and MDCK II cells. J Cell Sci 111(Pt 6):825–832
Williams TM, Lisanti MP (2004) The caveolin genes: from cell biology to medicine. Ann Med 36(8):584–595
Acknowledgments
Facilities and the scientific and technical assistance of the Australian Microscopy and Microanalysis Research Facility at the Australian Centre for Microscopy and Microanalysis, The University of Sydney. Bosch Advanced Microscope Unit, Bosch Institute, The University of Sydney. Molecular Biology Facility, Bosch Institute, The University of Sydney.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madawala, R.J., Dowland, S., Poon, C.E. et al. Caveolins redistribute in uterine epithelial cells during early pregnancy in the rat: An epithelial polarisation strategy?. Histochem Cell Biol 142, 555–567 (2014). https://doi.org/10.1007/s00418-014-1236-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-014-1236-8