Abstract
Purpose
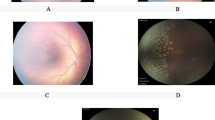
Stage 4A retinopathy of prematurity (ROP) is a critical phase where retinal detachment develops, but fovea is preserved. The present study aims to evaluate the effect of the first treatment choice (laser photocoagulation (LPC) or intravitreal ranibizumab (IVR)) applied in this critical phase on the prognosis of the disease.
Methods
Records of patients diagnosed with stage 4A ROP and whose first treatment was applied in our clinic were evaluated retrospectively. All patients were referred to our clinic for the treatment of advanced ROP . While group 1 was composed of the patients who were administered LPC as first treatment, group 2 included patients where IVR was applied as first treatment. The patients in both groups were referred to surgical treatment in the presence of progression.
Results
The present study included a total of 31 eyes in 16 patients with stage 4A ROP. Eighteen eyes of nine patients in group 1 were first applied LPC, and 13 eyes of seven patients in group 2 were first applied intravitreal ranibizumab. While anatomic outcomes of ten eyes in both groups were favorable, eight eyes in group 1 and three eyes in group 2 displayed progression and were referred to vitreoretinal surgery.
Conclusions
Laser and/or IVR treatment may be effective as a non-surgical treatment for stage 4A ROP. Especially stage 4A ROP until 6 clock hours can regress without surgical treatment. However, in stage 4A with involvement wider than 6 clock hours, non-surgical regression is difficult. Prospective controlled large series studies are necessary.

Similar content being viewed by others
References
International Committee for the Classification of Retinopathy of Prematurity (2005) The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 123:991–999
Futamura Y, Asami T, Nonobe N et al (2015) Buckling surgery and supplemental intravitreal bevacizumab or photocoagulation on stage 4 retinopathy of prematurity eyes. Jpn J Ophthalmol 59(6):378–388
El Rayes EN, Vinekar A, Capone A (2008) Three-year anatomic and visual outcomes after vitrectomy for stage 4B retinopathy of prematurity. Retina 28:568–572
Choi J, Kim JH, Kim S-J, Yu YS (2011) Long-term results of lens-sparing vitrectomy for stages 4B and 5 retinopathy of prematurity. Korean J Ophthalmol 25:305–310
Hartnett ME, Mccolm JR (2004) Retinal features predictive of progressive stage 4 retinopathy of prematurity. Retina 24(2):237–241
Hartnett ME, Rodier DW, McColm JR et al (2003) Long-term vision results measured with Teller acuity cards and a new light perception projection scale after management of late stages of retinopathy of prematurity. Arch Ophthalmol 121(7):991–996
Sato T, Kusaka S, Shimojo H, Fujikado T (2009) Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology 116:1599–1603
Sato T, Kusaka S, Shimojo H, Fujikado T (2009) Simultaneous analyses of vitreous levels of 27 cytokines in eyes with retinopathy of prematurity. Ophthalmology 116:2165–2169
Cheng HC, Lee SM, Hsieh YT, Lin PK (2015) Efficacy of intravitreal injection of anti-vascular endothelial growth factor agents for stage 4 retinopathy of prematurity. Retina 35(4):660–666
Zhou Y, Jiang Y, Bai Y, Wen JCL (2016) Vascular endothelial growth factor plasma levels before and after treatment of retinopathy of prematurity with ranibizumab. Graefes Arch Clin Exp Ophthalmol 254(1):31–36
Menke M, Framme C, Nelle M et al (2015) Intravitreal ranibizumab monotherapy to treat retinopathy of prematurity zone II, stage 3 with plus disease. BMC Ophthalmology;15–20
Early Treatment for Retinopathy of Prematurity Cooperative Group (2003) Revised indications for the treatment of retinopathy of prematurity: results of the Early Treatment for Retinopathy of Prematurity randomized trial. Arch Ophthalmol 121:1684–1694
Kychenthal A, Dorta P (2008) 25-gauge lens-sparing vitrectomy for stage 4A retinopathy of prematurity. Retina 28:S65–S68
Iwahashi-Shima C, Miki A, Hamasaki T et al (2012) Intraocular pressure elevation is a delayed-onset complication after successful vitrectomy for stages 4 and 5 retinopathy of prematurity. Retina 32:1636–1642
Hinz BJ, de Juan E Jr, Repka MX (1998) Scleral buckling surgery for active stage 4A retinopathy of prematurity. Ophthalmology 105:1827–1830
Chow DR, Ferrone PJ, Trese MT (1998) Refractive changes associated with scleral buckling and division in retinopathy of prematurity. Arch Ophthalmol 116:1446–1448
Repka MX, Tung B, Good WV, Capone A Jr, Shapiro MJ (2011) Outcome of eyes developing retinal detachment during the Early Treatment for Retinopathy of Prematurity study. Arch Ophthalmol 129(9):1175–1179
Krohne TU, Liu Z, Holz FG, Meyer CH (2012) Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am J Ophthalmol 154:682–686
Krohne TU, Eter N, Holz FG, Meyer CH (2008) Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol 146:508–512
Wu WC, Lien R, Liao PJ, Wang NK, Chen YP, Chao AN, Chen KJ, Chen TL, Hwang YS, Lai CC (2015) Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol 133:391–397
Kusaka S, Shima C, Wada K et al (2008) Efficacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study. Br J Ophthalmol 92:1450–1455
Wu WC, Yeh PT, Chen SN et al (2011) Effects and complications bevacizumab use in patients with retinopathy of prematurity: a multicenter study in Taiwan. Ophthalmology 118:176–183
Honda S, Hirabayashi H, Tsukahara Y, Negi A (2008) Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 246:1061–1063
Arevalo JF, Maia M, Flynn HW Jr et al (2008) Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol 92:213–216
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. The authors did not receive any financial support from any public or private sources. The authors have no financial or proprietary interest in a product, method, or material described herein.
Rights and permissions
About this article
Cite this article
Sukgen, E.A., Koçluk, Y. Treatment for stage 4A retinopathy of prematurity: laser and/or ranibizumab. Graefes Arch Clin Exp Ophthalmol 255, 263–269 (2017). https://doi.org/10.1007/s00417-016-3443-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3443-6