Abstract
Background
The combination of verteporfin photodynamic therapy (PDT) and anti-angiogenics has been shown to be safe and efficacious in the treatment of choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD). The purpose of this study is to demonstrate long-term prevention of vision loss and improvement in best-corrected visual acuity (BCVA) after treatment with one-time reduced-fluence-rate PDT followed by administration of ranibizumab on a variable dosing regimen over 24 months in patients with neovascular AMD. Secondary outcome measures included the change in central macular thickness (CMT), reinjection frequency, and safety.
Methods
This prospective, nonrandomized, open-label, single-center study enrolled 27 consecutive patients (27 eyes) presenting at the Leuven University Eye Hospital with previously untreated, active neovascular AMD between September 2006 and January 2007. All patients were treated with one-time, reduced-fluence-rate verteporfin PDT, followed by intravitreal ranibizumab 0.5 mg on the same day. A second and third ranibizumab injection were given at weeks 4 and 8, respectively, after which patients were followed up monthly for 24 months. Additional treatment with ranibizumab was administered to eyes with active neovascularization as indicated clinically and on imaging studies. Retreatment was based on the following criteria: (1) presence of subretinal fluid (SRF), intraretinal edema or sub-retinal pigment epithelial fluid, as seen on OCT; (2) increase of CMT by >100 mm on OCT; (3) signs of active CNV leakage on fluorescein angiography; (4) new sub- or intraretinal hemorrhage; and (5) BCVA decreased of ≥5 letters on the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart. If any single criterion for reinjection was fulfilled, retreatment with ranibizumab was administered.
Results
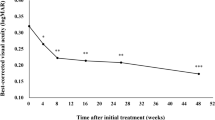
Twenty-five patients completed the 2-year study. Occult CNV was present in 64% and retinal angiomatous proliferative (RAP) lesions were present in 24% of the study eyes. The remaining three eyes had lesions classified as classic (one eye) or predominantly classic (two eyes) CNV. Month 24 data are available for 25 eyes (25 patients; age 55–86 years; mean 77; standard deviation (SD) = 7.2). Mean baseline VA was 58.6 letters (range: 35–70; SD = 8.4); 24-month VA was 66.2 letters (35–82; 12.7), not including one warfarin-treated patient who suffered vitreous hemorrhage. The mean visual acuity improved by 7.2 letters (p < 0.05) and the mean CMT decreased by 146 μm. VA improved >3 lines (15 letters) in 16%; improved 1–3 lines in 20%; remained within one line of baseline in 32%, decreased 1–3 lines in 16%, and decreased >3 lines in 16%. Losses of >3 lines were due to vitreous hemorrhage, geographic atrophy, fibrosis, and growth of an initially small CNV lesion. An average of 5.1 injections (range: 3–9) were administered during the first 12 months, and 7.1 injections (3–13) over 24 months. A total of 178 injections were performed; no systemic side-effects, uveitis, or choroidal collateral vascular damage were observed. Two patients were lost to follow-up.
Conclusion
Combined PDT and ranibizumab injection the same day was well tolerated in all patients. Eighty-four percent of patients had stable or improved vision at month 24.







Similar content being viewed by others
References
Zarbin MA (2004) Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol 122:598–614. doi:10.1001/archopht.122.4.598 122/4/598 [pii]
Kaiser PK, Brown DM, Zhang K, Hudson HL, Holz FG, Shapiro H, Schneider S, Acharya NR (2007) Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol 144:850–857. doi:S0002-9394(07)00718-0 [pii] 10.1016/j.ajo.2007.08.012
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T (2009) Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 116:57–65 e55. doi:S0161-6420(08)01075-0 [pii] 10.1016/j.ophtha.2008.10.018
Heier JS, Boyer DS, Ciulla TA, Ferrone PJ, Jumper JM, Gentile RC, Kotlovker D, Chung CY, Kim RY (2006) Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration: year 1 results of the FOCUS Study. Arch Ophthalmol 124:1532–1542. doi:124/11/1532 [pii] 10.1001/archopht.124.11.1532
Schmidt-Erfurth U, Wolf S (2008) Same-day administration of verteporfin and ranibizumab 0.5 mg in patients with choroidal neovascularisation due to age-related macular degeneration. Br J Ophthalmol 92:1628–1635. doi:bjo.2007.135277 [pii] 10.1136/bjo.2007.135277
Kiernan DF, Hariprasad SM, Chin EK, Kiernan CL, Rago J, Mieler WF (2009) Prospective comparison of Cirrus and Stratus optical coherence tomography for quantifying retinal thickness. Am J Ophthalmol 147:267–275 e262. doi:S0002-9394(08)00661-2 [pii] 10.1016/j.ajo.2008.08.018
Carpineto P, Nubile M, Toto L, Aharrh Gnama A, Marcucci L, Mastropasqua L, Ciancaglini M (2009) Correlation in foveal thickness measurements between spectral-domain and time-domain optical coherence tomography in normal individuals. Eye. doi:eye200976 [pii] 10.1038/eye.2009.76
Cukras C, Wang YD, Meyerle CB, Forooghian F, Chew EY, Wong WT (2009) Optical coherence tomography-based decision making in exudative age-related macular degeneration: comparison of time- vs spectral-domain devices. Eye. doi:eye2009211 [pii] 10.1038/eye.2009.211
Durbin M AT, Chang M, Lujan B (2009) Retinal measurements: comparison between Cirrus HD-OCT and Stratus OCT. Zeiss-Meditec White Paper
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431. doi:355/14/1419 [pii] 10.1056/NEJMoa054481
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355:1432–1444. doi:355/14/1432 [pii] 10.1056/NEJMoa062655
Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP, Shima DT (2007) Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol 171:53–67. doi:171/1/53 [pii]
Schlingemann RO (2004) Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 242:91–101. doi:10.1007/s00417-003-0828-0
Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, Shams N (2008) Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol 145:239–248. doi:S0002-9394(07)00881-1 [pii] 10.1016/j.ajo.2007.10.004
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, Puliafito CA, Davis JL, Flynn HW Jr, Esquiabro M (2007) An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 143:566–583. doi:S0002-9394(07)00068-2 [pii] 10.1016/j.ajo.2007.01.028
Holz FG, Korobelnik JF, Lanzetta P, Mitchell P, Schmidt-Erfurth U, Wolf S, Markabi S, Schmidli H, Weichselberger A (2009) The effects of a flexible visual acuity-driven ranibizumab treatment regimen in age-related macular degeneration: outcomes of a drug and disease model. Invest Ophthalmol Vis Sci. doi:iovs.09-3813 [pii] 10.1167/iovs.09-3813
Rosenfeld PJ, Rich RM, Lalwani GA (2006) Ranibizumab: phase III clinical trial results. Ophthalmol Clin North Am 19:361–372. doi:S0896-1549(06)00054-X [pii] 10.1016/j.ohc.2006.05.009
Spaide R (2007) Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol 143:679–680. doi:S0002-9394(07)00190-0 [pii] 10.1016/j.ajo.2007.02.024
Peters S, Julien S, Heiduschka P, Grisanti S, Ziemssen F, Adler M, Schraermeyer U, Bartz-Schmidt KU (2007) Antipermeability and antiproliferative effects of standard and frozen bevacizumab on choroidal endothelial cells. Br J Ophthalmol 91:827–831. doi:bjo.2006.109702 [pii] 10.1136/bjo.2006.109702
Volcker M, Peters S, Inhoffen W, Ziemssen F (2006) Early antiexudative response-OCT monitoring after intravitreal bevacizumab injection. Ophthalmologe 103:476–483. doi:10.1007/s00347-006-1356-1
Rosenfeld PJ, Moshfeghi AA, Puliafito CA (2005) Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 36:331–335
Bashshur ZF, Haddad ZA, Schakal A, Jaafar RF, Saab M, Noureddin BN (2008) Intravitreal bevacizumab for treatment of neovascular age-related macular degeneration: a one-year prospective study. Am J Ophthalmol 145:249–256. doi:S0002-9394(07)00854-9 [pii] 10.1016/j.ajo.2007.09.031
Kaiser PK, Blodi BA, Shapiro H, Acharya NR (2007) Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology 114:1868–1875. doi:S0161-6420(07)00467-8 [pii] 10.1016/j.ophtha.2007.04.030
Bradley J, Ju M, Robinson GS (2007) Combination therapy for the treatment of ocular neovascularization. Angiogenesis 10:141–148. doi:10.1007/s10456-007-9069-x
Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D (2003) Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest 111:1287–1295. doi:10.1172/JCI17929
Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K, Robinson GS, Adamis AP, Shima DT (2006) Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol 168:2036–2053. doi:168/6/2036 [pii]
D’Amore PA (1994) Mechanisms of retinal and choroidal neovascularization. Invest Ophthalmol Vis Sci 35:3974–3979
Casey R, Li WW (1997) Factors controlling ocular angiogenesis. Am J Ophthalmol 124:521–529
Das A, McGuire PG (2003) Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Prog Retin Eye Res 22:721–748. doi:S135094620300065X [pii]
Augustin A, Offerman I (2007) Combination therapy for choroidal neovascularisation. Drugs Aging 24:979–990
Schmidt-Erfurth UM, Richard G, Augustin A, Aylward WG, Bandello F, Corcostegui B, Cunha-Vaz J, Gaudric A, Leys A, Schlingemann RO (2007) Guidance for the treatment of neovascular age-related macular degeneration. Acta Ophthalmol Scand 85:486–494. doi:AOS979 [pii] 10.1111/j.1600-0420.2007.00979.x
Michels S, Schmidt-Erfurth U (2003) Sequence of early vascular events after photodynamic therapy. Invest Ophthalmol Vis Sci 44:2147–2154
Kaiser PK (2006) Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: 5-year results of two randomized clinical trials with an open-label extension: TAP report no. 8. Graefes Arch Clin Exp Ophthalmol 244:1132–1142. doi:10.1007/s00417-005-0199-9
Schmidt-Erfurth U, Schlotzer-Schrehard U, Cursiefen C, Michels S, Beckendorf A, Naumann GO (2003) Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium-derived factor. Invest Ophthalmol Vis Sci 44:4473–4480
Antoszyk AN, Tuomi L, Chung CY, Singh A (2008) Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol 145:862–874. doi:S0002-9394(08)00008-1 [pii] 10.1016/j.ajo.2007.12.029
Husain D, Kim I, Gauthier D, Lane AM, Tsilimbaris MK, Ezra E, Connolly EJ, Michaud N, Gragoudas ES, O’Neill CA, Beyer JC, Miller JW (2005) Safety and efficacy of intravitreal injection of ranibizumab in combination with verteporfin PDT on experimental choroidal neovascularization in the monkey. Arch Ophthalmol 123:509–516. doi:123/4/509 [pii] 10.1001/archopht.123.4.509
Azab M, Boyer DS, Bressler NM, Bressler SB, Cihelkova I, Hao Y, Immonen I, Lim JI, Menchini U, Naor J, Potter MJ, Reaves A, Rosenfeld PJ, Slakter JS, Soucek P, Strong HA, Wenkstern A, Su XY, Yang YC (2005) Verteporfin therapy of subfoveal minimally classic choroidal neovascularization in age-related macular degeneration: 2-year results of a randomized clinical trial. Arch Ophthalmol 123:448–457. doi:123/4/448 [pii] 10.1001/archopht.123.4.448
Singh CN, Saperstein DA (2008) Combination treatment with reduced-fluence photodynamic therapy and intravitreal injection of triamcinolone for subfoveal choroidal neovascularization in macular degeneration. Retina 28:789–793. doi:10.1097/IAE.0b013e31817082d7 00006982-200806000-00001 [pii]
Kramer M, Miller JW, Michaud N, Moulton RS, Hasan T, Flotte TJ, Gragoudas ES (1996) Liposomal benzoporphyrin derivative verteporfin photodynamic therapy. Selective treatment of choroidal neovascularization in monkeys. Ophthalmology 103:427–438
Miller JW, Schmidt-Erfurth U, Sickenberg M, Pournaras CJ, Laqua H, Barbazetto I, Zografos L, Piguet B, Donati G, Lane AM, Birngruber R, van den Berg H, Strong A, Manjuris U, Gray T, Fsadni M, Bressler NM, Gragoudas ES (1999) Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: results of a single treatment in a phase 1 and 2 study. Arch Ophthalmol 117:1161–1173
Michels S, Hansmann F, Geitzenauer W, Schmidt-Erfurth U (2006) Influence of treatment parameters on selectivity of verteporfin therapy. Invest Ophthalmol Vis Sci 47:371–376. doi:47/1/371 [pii] 10.1167/iovs.05-0354
Acknowledgements
The authors would like to thank Carol Heughebaert for her editorial assistance and Adrian Loehwing for her help with the statistical analysis. This study was supported by Novartis Inc, Basel, Switzerland, and the Department of Ophthalmology at the Leuven University Hospital, Leuven, Belgium. Dr. Leys has received research grants from Novartis Inc, has participated in competing scientific advisory boards, and has received honorarium and reimbursement for travel expenses from Novartis. We thank the following: those involved in design of study (A.L.); conduct of study (A.L., L.S.); data collection (L.S., A.L.); management (L.S., A.L.); analysis (L.S., A.L), and interpretation of data (L.S., A.L.); and preparation (L.S.), review and approval of the manuscript (L.S., A.L.). Before the initiation of the study, approval to perform the TORPEDO Study was obtained from the Institutional Review Board at the Leuven University Hospital. The study was registered with https://eudract.emea.europa.eu (no. 2006-003976-36). The study adhered to the tenets of the Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Additional information
Novartis supplied Lucentis for the study.
The authors have full control of all primary data and they agree to allow Graefe’s Archive for Clinical and Experimental Ophthalmology to review their data if requested.
This clinical trial was approved by and registered with the Ethics Committee of the Catholic University of Leuven on May 23, 2006.
EudraCT number: 2006-003976-36
Sponsor’s protocol code number: CBPD952ABE02
The results of this trial were shared in a paper presented at the Association for Research in Vision and Ophthalmology (ARVO) annual meeting, Fort Lauderdale, Florida, USA, on May 7, 2009.
Rights and permissions
About this article
Cite this article
Spielberg, L., Leys, A. Treatment of neovascular age-related macular degeneration with a variable ranibizumab dosing regimen and one-time reduced-fluence photodynamic therapy: the TORPEDO trial at 2 years. Graefes Arch Clin Exp Ophthalmol 248, 943–956 (2010). https://doi.org/10.1007/s00417-009-1256-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-009-1256-6