Abstract
Purpose
Lung injury can be caused by ventilation and non-physiological lung stress (transpulmonary pressure) and strain [inflated volume over functional residual capacity ratio (FRC)]. FRC is severely decreased in patients with acute respiratory distress syndrome (ARDS). End-expiratory lung volume (EELV) is FRC plus lung volume increased by the applied positive end-expiratory pressure (PEEP). Measurement using the modified nitrogen multiple breath washout technique may help titrating PEEP during ARDS and allow determining dynamic lung strain (tidal volume over EELV) in patients ventilated with PEEP. In this observational study, we measured EELV for up to seven consecutive days in patients with ARDS at different PEEP levels.
Results
Thirty sedated patients with ARDS (10 mild, 14 moderate, 6 severe) underwent decremental PEEP testing (20, 15, 10, 5 cm H2O) for up to 7 days after inclusion. At all PEEP levels examined, over a period of 7 days the measured absolute EELVs showed no significant change over time [PEEP 20 cm H2O 2464 ml at day 1 vs. 2144 ml at day 7 (p = 0.78), PEEP 15 cm H2O 2226 ml vs. 1990 ml (p = 0.36), PEEP 10 1835 ml vs. 1858 ml (p = 0.76) and PEEP 5 cm H2O 1487 ml vs. 1612 ml (p = 0.37)]. In relation to the predicted body weight (pbw), no significant change in EELV/kg pbw over time could be detected either at any PEEP level or over time [PEEP 20 36 ml/kg pbw at day 1 vs. 33 ml/kg pbw at day 7 (p = 0.66); PEEP 15 33 vs. 29 ml/kg pbw (p = 0.32); PEEP 10 27 vs. 27 ml/kg pbw (p = 0.70) and PEEP 5 22 vs. 24 ml/kg pbw (p = 0.70)]. Oxygenation significantly improved over time from PaO2/FiO2 of 169 mmHg at day 1 to 199 mmHg at day 7 (p < 0.01).
Conclusions
EELV did not change significantly for up to 7 days in patients with ARDS. By contrast, PaO2/FiO2 improved significantly. Bedside measurement of EELV may be a novel approach to individualise lung-protective ventilation on the basis of calculation of dynamic strain as the ratio of VT to EELV.
Similar content being viewed by others
Introduction
It is a well-known fact that mechanical ventilation can cause injury to the lung of patients with acute respiratory distress syndrome (ARDS). Strategies to reduce this ventilator-associated lung injury (VALI) include limitation of plateau pressure, low tidal volume (VT) and positive end-expiratory pressure (PEEP) titration [1–3]. Gattinoni et al. introduced the concept of stress (transpulmonary pressure) and strain (ratio of VT to functional residual capacity (FRC)) which reflect the external forces affecting the mechanically ventilated lungs [4, 5]. They postulated a threshold value beyond which both stress and strain are harmful [6]. Strain could be broken down into dynamic strain and static strain. In mechanical ventilation without PEEP, strain can be calculated as the ratio between VT and FRC. When an external PEEP is applied in mechanical ventilation, the lungs will be kept tonically inflated above their FRC (VPEEP) [7]. This dynamic strain can be calculated as VT over EELV and correct the strain for alveolar recruitment.
ARDS leads to a substantial decrease in EELV, leading to a higher strain at a given VT [4,8,9]. These high strain levels are associated with several alterations with potential harmful effects which have been addressed in some recent studies. There is a clear association between high strain and VALI [6]. Increased strain is associated with a pro-inflammatory lung response in patients with acute lung injury [10]. Moreover, regional strain is associated with increasing metabolic activity of lung tissue in animal and human studies in ARDS [11, 12]. Recently, Amato et al. [13] could show in an analysis of 3562 patients with ARDS enrolled in nine previously reported randomised trials that driving pressure, defined as the ratio of VT to respiratory-system compliance (CRS), was strongly associated with survival. The ratio of VT to CRS is nothing else than an approximation of strain (VT/EELV). Therefore, a reduction of strain by an optimal VT and PEEP management may have a lung-protective approach [14].
Interestingly, little is known about how EELV changes in the clinical course of patients with ARDS treated on an intensive care unit. We measured EELV at the bedside without interruption of mechanical ventilation using the modified nitrogen multiple breath washout (NMBW) technique [15], which is integrated into an intensive care ventilator. To analyse the influence of PEEP on EELV over time, we measured EELV during a daily decremental PEEP trial (20, 15, 10, 5 cm H2O) for up to 7 days after study inclusion in patients with ARDS.
Patients and Methods
This prospective study was conducted, with approval of the Ethics Committee of the Medical Department Mannheim of Heidelberg University, between October 2011 and June 2013 at an intensive care unit of the university. After due information, written consent to study participation had been obtained from the participating patients or their family members, which could be revoked without giving reasons. The ICU acts as a tertiary care centre for ARDS.
All consecutive adult patients with ARDS according to standard criteria [16, 17] were examined for possible inclusion into the study. Exclusion criteria were ARDS present for more than 72 h, start of ventilation more than 72 h ago, severe haemodynamic instability preventing application of a PEEP of 20 cm H2O, an FiO2 ≥ 0.8, an extracorporeal procedure (ECMO or pECLA), use of high-frequency oscillation ventilation (HFOV), abnormal airway anatomy due to partial lung resection or fistulas.
All patients were ventilated in BiLevel or CPAP mode on the first day of the examination. Inspiratory pressure was controlled so that a tidal volume of 6 ml/kg predicted body weight (pbw) [1] was reached. Before the start of each measurement, in order to ensure identical examination conditions, it was checked that the respective patient had been in supine position for at least 15 min. Patients were sedated not paralysed.
Study Protocol
-
1.
Preparation: First, the currently selected ventilation parameters (ventilation mode, PEEP, FiO2), the tidal volume resulting from the airway pressures were recorded.
-
2.
Recruitment: For this purpose, PEEP was first adjusted to 20 cm H2O. Thereafter, inspiratory pressure was adjusted so that an upper pressure level of 45 cm H2O was reached. Using the “Inspiratory stop” function of the respirator, an upper pressure level of 45 cm H2O was maintained for 15 s. Then PEEP was maintained at 20 cm H2O and inspiratory pressure adjusted to ensure a VT of 6 ml/kg pbw. During the subsequent measurements, inspiratory pressure was not changed.
-
3.
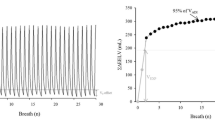
EELV measurement at a PEEP of 20 cm H2O: The patient was ventilated for at least 15 min at a PEEP of 20 cm H2O. Achieving a steady-state situation by means of a stable carbon dioxide volume (\({\text{V}}_{{{\text{CO}}_{2} }}\)) for at least 10 min is essential for EELV measurement, as \({\text{V}}_{{{\text{CO}}_{2} }}\) is one of the main parameters for EELV calculation [15]. Therefore, patients with an extracorporeal CO2 elimination device were not included. EELV measurement was started on the Engström Carestation® respirator. We measured EELV using the NMBW technique, described in detail elsewhere [15]. The respirator was equipped with a COVX module providing the data for EELV calculation. For EELV measurement, we used a step change in FiO2 of 0.2. EELV at each PEEP and measured twice (wash-out and wash-in). At a FiO2 increase of 0.2, each complete wash-out and wash-in cycle took about 40 breaths, so that one measurement was completed within 10 min. After completion of the measurement programme at a PEEP of 20 cm H2O, PEEP was reduced to 15 cm H2O, and inspiratory pressure was maintained.
-
4.
Measurement at a PEEP of 15 cm H2O: in analogy to item 3, followed by PEEP reduction to 10 cm H2O.
-
5.
Measurement at a PEEP of 10 cm H2O: in analogy to item 3, followed by PEEP reduction to 5 cm H2O.
The test protocol in items 1–5 was conducted up to the seventh day after enrolment of the patient.
We measured EELV as an absolute value as calculated by the ventilator. This absolute EELV was then normalised by dividing the predicted body weight of the patient.
Strain was calculated as the ratio of the applied TV to the absolute EELV.
Measurement Errors and Cancellations
Whenever a patient would not tolerate the changes in ventilation parameters prescribed in the measurement cycle and a single measurement could not be completed successfully, the cause was documented.
Respiratory reasons: Drop in arterial oxygen saturation >10 %, decrease in tidal volume >20 %; cardio-circulatory reasons: Drop in mean arterial pressure >20 %, change in heart rate >20 %; measurement error: if five consecutive measurements were aborted by the device, this was documented as a measurement error.
Premature Study Exclusions
Reasons for Exclusion of a Patient Prior to Expiry of the 7-Day Study Protocol
Extubation, death, escalation of the ventilation therapy to HFOV, pECLA or ECMO. Persistent haemodynamic instability preventing measurements for several days.
Statistical Analysis
The collected data were analysed using the SAS software, Release 9.3 (SAS Institute Inc., Cary, NC, USA). For quantitative variables, mean values and standard deviations were calculated.
To determine whether these collected values changed at the individual PEEP levels in the course of the measurement period of 7 days, an analysis of variance for repeated measurements (ANOVA) was performed for each parameter collected using the SAS procedure PROC MIXED regarding the day as a fixed and the patients’ ID as a random factor. If here global comparison of the mean values of all days yielded a significant result, the data from days 2 to 7 were in each case compared with those from day 1 using Dunnett’s test. In case of a global p value >0.10, it was assumed that the parameter in question does not change significantly over the course of 7 days, and the pairwise comparisons were waived.
The dependence of the parameters EELV, EELV relative to pbw and PaO2/FiO2 on the respectively selected PEEP was checked by means of the Wilcoxon signed-rank test for paired groups, which examines the differences of the central trend of two related samples. For this purpose, per study participant and parameter, the median values from all measurement days at one PEEP level were compared pairwise to those of the immediately lower PEEP level, thus e.g. average EELV at a PEEP of 20 cm H2O versus EELV at a PEEP of 15 cm H2O.
The correlation between EELV and PaO2/FiO2 was calculated using the Spearman test.
For all statistical tests, the significance level was set to 5 %.
The results are shown as mean ± standard deviation (SD).
Results
Thirty orotracheally intubated and sedated patients (mean age 57.6 ± 18.6; 16 male, 14 female) were examined.
At the time of the first measurement, the PaO2/FiO2 ratio was 169.23 ± 69.56 mmHg. In 10 patients, it was mild, in 14 moderate and in 6 severe ARDS according to the Berlin definition. The PEEP selected by the medical ward staff was 13.8 ± 3.51 cm H2O. The period from the beginning of an invasive or non-invasive ventilation measure until the first measurement of EELV according to the protocol ranged from 6 to 70 h (Tables 1, 2).
Readings Over Time
At all PEEP levels examined, over a period of 7 days the measured absolute EELVs showed no significant change (PEEP 20 (p = 0.78), PEEP 15 (p = 0.36), PEEP 10 (p = 0.76) and PEEP 5 cm H2O (p = 0.37)) (Fig. 1) (Table 3). In relation to predicted body weight, no significant change in EELV over time could be detected either at any PEEP level (PEEP 20: p = 0.66; PEEP 15: p = 0.32; PEEP 10: p = 0.70 and PEEP 5: p = 0.70) (Fig. 2). Overall, 528 measurements were successfully completed.
Comparison of the median values of the measured absolute EELVs and EELVs in relation to the predicted body weight of each study participant across all measurement days showed a statistically significant correlation with the PEEP applied during measurement: The volumes decreased in the same direction as PEEP (Tables 4, 5, Supplementary data).
The PEEP selected by the ward staff on the respective measurement day was on average 13.8 ± 3.51 cm H2O on day 1 and 11.36 ± 3.25 cm H2O on day 7 (p < 0.0001) (Table 3). The PaO2/FiO2 ratio measured at this PEEP level was on average 169 ± 70 mmHg on day 1 and 199 ± 54 mmHg on day 7 (p < 0.01) (Table 3). Although we found a significant correlation between PaO2/FiO2 and EELV, the correlation was very weak, with r = 0.31 for all analysed 520 pairs.
Premature Exclusions and Aborted Measurements
In a total of 16 patients, the measurements had to be terminated before the end of the 7 days specified in the study protocol: Nine could be extubated, five deceased, in one therapy had to be escalated to HFOV and in one the haemodynamic condition on the last study day allowed no measurement.
Of the resulting total of 622 measurements begun, 94 measurements at various PEEP levels had to be aborted—65 due to respiratory and 24 due to cardiocirculatory reasons, and another 5 due to unrecoverable measurement errors. This corresponds to 15 % of the 622 measurements begun.
Discussion
This is the first study that examines EELV in patients with ARDS over a period of 7 days.
In the course of 1 week, no statistically significant change in EELV over time was seen in the study population at any of the four PEEP levels of 20, 15, 10 and 5 cm H2O.
So far, the high expenditures for equipment and staff of most methods for EELV determination have hampered a systematic follow-up of this parameter in severely ill ICU patients. The MBNW (multiple breath nitrogen washout) method developed by Olegard et al. [15] has now been implemented in the form of commercially available devices that allow such measurements to be performed at the bedside, without having to disconnect the patient from the intensive respirator, to change the ventilation parameters (apart from a minor FiO2 adjustment) or to perform repositioning and transport measures.
In the context of single measurement, numerous studies have shown that bedside measurement by means of the MBNW technique yields reproducible results even in ARDS patients [4, 8, 9, 18]. Since the positioning of the patients affects their EELVs, all measurements were carried out in supine position [19].
In all patients, after an initial recruitment manoeuvre, we performed the measurements in terms of a decremental PEEP trial [18]. Since we wanted to examine patients with ARDS of varying severities, we limited the lowest PEEP value to 5 cm H2O. We analysed the values of EELV for up to 7 days in a fixed manner. We did not measure the FRC at a PEEP level of zero, nor at the “optimal” PEEP level of each individual patient. The medical team titrated the PEEP from 13.8 cm H2O at study entry down to 11.4 cm H2O on day 7, reflecting an individual approach to an optimal PEEP for the patient.
Dependence of EELV on PEEP was demonstrated both in animal models and in patients [8, 20, 21]. In their study published in 2008, Bikker et al. [21] examined the PEEP dependence of EELV and found a correlation similar to that described in our investigation. They divided their 45-patient study population into pulmonary healthy, primarily lung-injured and secondarily lung-injured patients. Interestingly, they found a correlation between EELV and paO2/FiO2 only in the subgroup of 16 primarily lung-injured patients. In our study, we found a statistically significant correlation between EELV and PaO2/FiO2, but the correlation was very weak, with a correlation index r of 0.31. Inverse correlation between arterial oxygenation and the amount of collapsed lung mass has been found in CT scan studies in patients with ARDS [22]. However, only frail correlation between PEEP-induced recruitment and arterial oxygenation has been documented in other studies [23, 24]. As alveolar recruitment and lung over-inflation can be simultaneously observed, changes in PaO2 should not be considered as a sensitive parameter to detect the risk of VALI [25]. In an animal study with acute lung injury, only weak correlation (r 2 = 0.53) of EELV and PaO2 was found [20]. In our study, the increase in PEEP resulted in a significant increase in EELV and thus, theoretically, in a decrease in strain at a given VT. But it has to be pointed out that the isolated consideration of an increase in EELV does not directly imply strain reduction. As shown in Tables 5 and 6 (supplementary data), the increase of EELV from a PEEP of 15 to a PEEP of 20 was not as big as the increase from PEEP 5 to PEEP 10. This suggests that over-distension occurred, rather than recruitment. In terms of an individual approach to optimal PEEP, this means that the PEEP should titrate to larger increase in EELV compared to the increase in PEEP. Measurement of EELV was a more sensitive indicator of PEEP-induced aeration and recruitment than compliance in a model of experimental lung injury [26].
Interestingly, in our study population at none of the four PEEP levels of 20, 15, 10 and 5 cm H2O, a statistically significant change in EELV was seen over the course of 7 days. By contrast, PaO2/FiO2 improved significantly until day 7 of the investigation. These results have not been published elsewhere yet. Thus, it may be concluded that oxygenation should not be used for selection of a ventilation regime under the aspect of lung-protective ventilation. The reasons why oxygenation improves over time, although EELV is still reduced, remain speculative. Obviously, other factors in addition to ventilation management, e.g. haemodynamic optimisation or control of infection, may lead to that improvement.
Strain in ARDS
The concept of stress and strain during mechanical ventilation was developed by Gattinoni et al. [5, 6, 27]. To describe the drivers of stress on lung parenchyma, the parameters stress, specific elastance and strain were proposed (stress = specific elastance × strain).
Chiumello et al. [27] calculated lung stress and strain in 80 volume-controlled ventilated patients with and without lung disorders, at two different PEEP levels (5 and 15 cm H2O) and four different tidal volumes (6, 8, 10 and 12 ml/kg pbw). Stress was calculated based on oesophageal pressure, whereas EELV (strain) was measured using a balloon with helium when mechanical ventilation was interrupted for each measurement. They conclude that VT levels based on pbw and airway plateau pressure were inadequate surrogates for lung stress and strain [27].
The relevance of lung volume as a component of VALI, measured as VT/EELV, has recently been shown in studies on humans [10, 11] and animals [6]. Gonzalez-Lopez studied 22 patients with acute lung injury and control patients [10]. Patients were divided into one normal-strain and one high-strain group. Patients with acute lung injury and strain >0.27 showed significantly more inflammatory cytokines in the broncho-alveolar lavage fluid than control subjects or patients with acute lung injury and normal strain. Therefore, it may be postulated that dynamic strain of <0.27 should be sought in mechanically ventilated patients. Bellani et al. [11] estimated metabolic activity in aerated parts of the lungs of patients with acute lung injury and found an increase of metabolic activity with increasing strain. Calculation of strain based on a bedside measurement of EELV may therefore be suitable for individualising VT to a more protective value.
Several studies have demonstrated the accuracy and precision of the NMBW technique to measure EELV at the bedside in mechanical ventilated patients, even in patients and animal models with ARDS [8, 9, 23, 28, 29]. Nonetheless, some issues have to be pointed out. Richard et al. [25] assessed the reliability of the NMBW technique at different PEEP and VT levels in a saline lavage model on 14 piglets. Functional residual capacity (FRC, at zero end-expiratory pressure) was very similar to the CT-scan assessment, whereas the NMBW technique underestimates EELV at high PEEP up to 20 cm H2O and VT up to 10 ml/kg in this experimental model of ARDS. The observed differences in EELV at high PEEP may be related to the fact that the NMBW technique measures functional, i.e. ventilated, lung regions, while CT measures aeration of both ventilated and non-ventilated regions. In our study, we used a fixed VT of 6 ml/kg pbw, which should allow an accurate estimation of EELV. Patroniti et al. [30] suggested that the PEEP-induced recruited lung volume above the FRC may be underestimated when using higher PEEP, as the “true” FRC may be modified by PEEP. Therefore, the absolute value of EELV may be underestimated in our study as we were not using a long exhalation to zero PEEP.
In conclusion, measurement of EELV in patients with ARDS can be reliably done and provide data for calculating pulmonary strain at the bedside in critically ill patients. No significant change in EELV, neither in absolute values nor in relation to predicted body weight over time, could be detected at any PEEP level. As dynamic strain calculated as the ratio between VT and EELV is persistent high for up to 7 days after inclusion, bedside measurement of EELV might be a novel approach to individualise lung-protective ventilation based on an optimal VT and PEEP.
References
(2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Amato MBP, Barbas CSV, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CRR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
Meade MO, Cook DJ, Guyatt GH, Al E (2008) Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299:637–645
Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, Tallarini F, Cozzi P, Cressoni M, Colombo A, Marini JJ, Gattinoni L (2008) Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med 178:346–355
Gattinoni L, Protti A, Caironi P, Carlesso E (2010) Ventilator-induced lung injury: the anatomical and physiological framework. Crit Care Med 38:S539–S548
Protti A, Cressoni M, Santini A, Langer T, Mietto C, Febres D, Chierichetti M, Coppola S, Conte G, Gatti S, Leopardi O, Masson S, Lombardi L, Lazzerini M, Rampoldi E, Cadringher P, Gattinoni L (2011) Lung stress and strain during mechanical ventilation. Am J Respir Crit Care Med 183:1354–1362
Protti A, Votta E, Gattinoni L (2014) Which is the most important strain in the pathogenesis of ventilator-induced lung injury: dynamic or static? Curr Opin Crit Care 20:33–38
Dellamonica J, Lerolle N, Sargentini C, Beduneau G, Di Marco F, Mercat A, Richard JC, Diehl JL, Mancebo J, Rouby JJ, Lu Q, Bernardin G, Brochard L (2011) Accuracy and precision of end-expiratory lung-volume measurements by automated nitrogen washout/washin technique in patients with acute respiratory distress syndrome. Crit Care 15:R294
Dellamonica J, Lerolle N, Sargentini C, Beduneau G, Di Marco F, Mercat A, Richard JC, Diehl JL, Mancebo J, Rouby JJ, Lu Q, Bernardin G, Brochard L (2011) PEEP-induced changes in lung volume in acute respiratory distress syndrome. Two methods to estimate alveolar recruitment. Intensive Care Med 37:1595–1604
González-López A, García-Prieto E, Batalla-Solís E, Amado-Rodríguez L, Avello N, Blanch L, Albaiceta G (2012) Lung strain and biological response in mechanically ventilated patients. Intensive Care Med 38:240–247
Bellani G, Guerra L, Musch G, Zanella A, Patroniti N, Mauri T, Messa C, Pesenti A (2011) Lung regional metabolic activity and gas volume changes induced by tidal ventilation in patients with acute lung injury. Am J Respir Crit Care Med 183:1193–1199
Wellman TJ, Winkler T, Costa ELV, Al E (2014) Effect of local tidal lung strain on inflammation in normal and lipopolysaccharide-exposed sheep. Critical care medicine. Crit Care Med 42:e491–e500
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG (2015) Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372:747–755
Chen L, Brochard L (2015) Lung volume assessment in acute respiratory distress syndrome. Curr Opin Crit Care 21:259–264
Olegard C, Sondergaard S, Houltz E, Lundin S, Stenqvist O (2005) Estimation of functional residual capacity at the bedside using standard monitoring equipment: a modified nitrogen washout/washin technique requiring a small change of the inspired oxygen fraction. Anesth Analg 101:206–212
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R (1994) Report of the American–European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med 20:225–232
Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA 307:2526–2533
Blankman P, Hasan D, Bikker IG, Gommers D (2015) Lung stress and strain calculations in mechanically ventilated patients in the intensive care unit. Acta Anaesthesiol Scand. doi:10.1111/aas.12589
Dellamonica J, Lerolle N, Sargentini C, Hubert S, Beduneau G, Di Marco F, Mercat A, Diehl JL, Richard JC, Bernardin G, Brochard L (2013) Effect of different seated positions on lung volume and oxygenation in acute respiratory distress syndrome. Intensive Care Med 39:1121–1127
Lambermont B, Ghuysen A, Janssen N, Morimont P, Hartstein G, Gerard P, D’orio V (2008) Comparison of functional residual capacity and static compliance of the respiratory system during a positive end-expiratory pressure (PEEP) ramp procedure in an experimental model of acute respiratory distress syndrome. Crit Care 12:R91
Bikker IG, Leonhardt S, Bakker J, Gommers D (2009) Lung volume calculated from electrical impedance tomography in ICU patients at different PEEP levels. Intensive Care Med 35:1362–1367
Borges JB, Okamoto VN, Matos GFJ, Caramez MPR, Arantes PR, Barros F, Souza CE, Victorino JA, Kacmarek RM, Barbas CSV, Carvalho CRR, Amato MBP (2006) Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. AJRCCM 174:268–278
Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ, C. T. Scan ARDS Study Group (2001) Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. AJRCCM 163:1444–1450
Maggiore SM, Jonson B, Richard J-C, Jaber S, Lemaire F, Brochard L (2001) Alveolar derecruitment at decremental positive end-expiratory pressure levels in acute lung injury. AJRCCM 164:796–801
Rouby JJ, Lu Q, Goldstein I (2002) Selecting the right level of positive end expiratory pressure in patients with acute respiratory distress syndrome. AJRCCM 165:1182–1186
Rylander C, Hogman M, Perchiazzi G, Magnusson A, Hedenstierna G (2004) Functional residual capacity and respiratory mechanics as indicators of aeration and collapse in experimental lung injury. Anesth Analg 98:782–789
Chiumello D, Cressoni M, Chierichetti M, Tallarini F, Botticelli M, Berto V, Mietto C, Gattinoni L (2008) Nitrogen washout/washin, helium dilution and computed tomography in the assessment of end expiratory lung volume. Crit Care 12:R150
Bikker IG, Van Bommel J, Miranda DR, Bakker J, Gommers D (2008) End-expiratory lung volume during mechanical ventilation: a comparison with reference values and the effect of positive end-expiratory pressure in intensive care unit patients with different lung conditions. Crit Care 12:R145
Richard J-C, Maggiore SM, Mercat A (2004) Clinical review: bedside assessment of alveolar recruitment. Crit Care 8:163–169
Patroniti N, Bellani G, Manfio A, Maggioni E, Giuffrida A, Foti G, Pesenti A (2004) Lung volume in mechanically ventilated patients: measurement by simplified helium dilution compared to quantitative CT scan. Intensive Care Med 30:282–289
Authors’ Contributions
AK designed the study, performed experimental research, compiled data tables/figures and drafted/revised the manuscript. FG performed experimental research, compiled data tables/figures and drafted/revised the manuscript. CW performed the statistical analysis, compiled data tables/figures and drafted/revised the manuscript. TV designed the study and drafted/revised the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Armin Kalenka has been a consultant for GE Healthcare and received lecture fees. The other authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kalenka, A., Gruner, F., Weiß, C. et al. End-Expiratory Lung Volume in Patients with Acute Respiratory Distress Syndrome: A Time Course Analysis. Lung 194, 527–534 (2016). https://doi.org/10.1007/s00408-016-9892-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-016-9892-1