Abstract
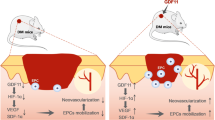
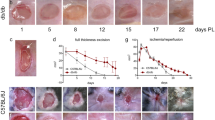
Mast cells (MCs) reside in cutaneous tissue, and an increment of MCs is suggested to induce vascular regression in the process of wound healing. To clarify participation of MCs in diabetic cutaneous wound healing, we created an excisional wound on diabetic mice 4 weeks after streptozotocin injections and subsequently investigated the healing processes for 49 days, comparing them with control mice. The rate of wound closure was not markedly different between the diabetic and control mice. In the proliferative phase at days 7 and 14, neovascularization in the wound was weaker in diabetic mice than in control mice. In the remodeling phase at day 21 and afterward, rapid vascular regression occurred in control mice; however, neovascularization was still observed in diabetic mice where the number of vessels in granulation tissues was relatively higher than in control mice. In the remodeling phase of the control mice, MCs within the wound began to increase rapidly and resulted in considerable accumulation, whereas the increment of MCs was delayed in diabetic mice. In addition, the number of fibroblast growth factor (FGF)- or vascular endothelial growth factor (VEGF)-immunopositive hypertrophic fibroblast-like spindle cells and c-Kit-positive/VEGFR2-positive/FcεRIα-negative endothelial progenitor cells (EPCs) were higher in diabetic wounds. In conclusion, neovascularization in the proliferative phase and vascular regression in the remodeling phase were impaired in diabetic mice. The delayed increment of MCs and sustained angiogenic stimuli by fibroblast-like spindle cells and EPCs may inhibit vascular regression in the remodeling phase and impair the wound-healing process in diabetic mice.








Similar content being viewed by others
References
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341:738–746
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453:314–321
Iruela-Arispe ML, Dvorak HF (1997) Angiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Haemost 78:672–677
Peplow PV, Baxter GD (2012) Gene expression and release of growth factors during delayed wound healing: a review of studies in diabetic animals and possible combined laser phototherapy and growth factor treatment to enhance healing. Photomed Laser Surg 30:617–636
Tahergorabi Z, Khazaei M (2012) Imbalance of angiogenesis in diabetic complications: the mechanisms. Int J Prev Med 3:827–838
Martin A, Komada MR, Sane DC (2003) Abnormal angiogenesis in diabetes mellitus. Med Res Rev 23:117–145
Keswani SG, Katz AB, Lim FY, Zoltick P, Radu A, Alaee D, Herlyn M, Crombleholme TM (2004) Adenoviral mediated gene transfer of PDGF-B enhances wound healing in type I and type II diabetic wounds. Wound Repair Regen 12:497–504
Kwon DS, Gao X, Liu YB, Dulchavsky DS, Danyluk AL, Bansal M, Chopp M, McIntosh K, Arbab AS, Dulchavsky SA, Gautam SC (2008) Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. Int Wound J 5:453–463
Maharlooei MK, Bagheri M, Solhjou Z, Jahromi BM, Akrami M, Rohani L, Monabati A, Noorafshan A, Omrani GR (2011) Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Res Clin Pract 93:228–234
Starkey JR, Crowle PK, Taubenberger S (1988) Mast-cell-deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer 42:48–52
Noli C, Miolo A (2001) The mast cell in wound healing. Vet Dermatol 12:303–313
Trautmann A, Toksoy A, Engelhardt E, Brocker EB, Gillitzer R (2000) Mast cell involvement in normal human skin wound healing: expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J Pathol 190:100–106
Egozi EI, Ferreira AM, Burns AL, Gamelli RL, Dipietro LA (2003) Mast cells modulate the inflammatory but not the proliferative response in healing wounds. Wound Repair Regen 11:46–54
Antsiferova M, Martin C, Huber M, Feyerabend TB, Forster A, Hartmann K, Rodewald HR, Hohl D, Werner S (2013) Mast cells are dispensable for normal and activin-promoted wound healing and skin carcinogenesis. J Immunol 191:6147–6155
Nauta AC, Grova M, Montoro DT, Zimmermann A, Tsai M, Gurtner GC, Galli SJ, Longaker MT (2013) Evidence that mast cells are not required for healing of splinted cutaneous excisional wounds in mice. PLoS One 8:e59167
Willenborg S, Eckes B, Brinckmann J, Krieg T, Waisman A, Hartmann K, Roers A, Eming SA (2014) Genetic ablation of mast cells redefines the role of mast cells in skin wound healing and bleomycin-induced fibrosis. J Invest Dermatol. doi:10.1038/jid.2014.12
Nishikori Y, Kakizoe E, Kobayashi Y, Shimoura K, Okunishi H, Dekio S (1998) Skin mast cell promotion of matrix remodeling in burn wound healing in mice: relevance of chymase. Arch Dermatol Res 290:553–560
Shiota N, Nishikori Y, Kakizoe E, Shimoura K, Niibayashi T, Shimbori C, Tanaka T, Okunishi H (2010) Pathophysiological role of skin mast cells in wound healing after scald injury: study with mast cell-deficient W/W(V) mice. Int Arch Allergy Immunol 151:80–88
Kim KL, Meng Y, Kim JY, Baek EJ, Suh W (2011) Direct and differential effects of stem cell factor on the neovascularization activity of endothelial progenitor cells. Cardiovasc Res 92:132–140
Dentelli P, Rosso A, Balsamo A, Colmenares Benedetto S, Zeoli A, Pegoraro M, Camussi G, Pegoraro L, Brizzi MF (2007) C-KIT, by interacting with the membrane-bound ligand, recruits endothelial progenitor cells to inflamed endothelium. Blood 109:4264–4271
Lan CC, Wu CS, Kuo HY, Huang SM, Chen GS (2009) Hyperglycaemic conditions hamper keratinocyte locomotion via sequential inhibition of distinct pathways: new insights on poor wound closure in patients with diabetes. Br J Dermatol 160:1206–1214
Michaels J, Churgin SS, Blechman KM, Greives MR, Aarabi S, Galiano RD, Gurtner GC (2007) Db/db mice exhibit severe wound-healing impairments compared with other murine diabetic strains in a silicone-splinted excisional wound model. Wound Repair Regen 15:665–670
Trousdale RK, Jacobs S, Simhaee DA, Wu JK, Lustbader JW (2009) Wound closure and metabolic parameter variability in a db/db mouse model for diabetic ulcers. J Surg Res 151:100–107
Greenhalgh DG (2003) Tissue repair in models of diabetes mellitus: a review. Methods Mol Med 78:181–189
Mirza R, Koh TJ (2011) Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 56:256–264
Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ (2004) Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 53:195–199
Fiorina P, Pietramaggiori G, Scherer SS, Jurewicz M, Mathews JC, Vergani A, Thomas G, Orsenigo E, Staudacher C, La Rosa S, Capella C, Carothers A, Zerwes HG, Luzi L, Abdi R, Orgill DP (2010) The mobilization and effect of endogenous bone marrow progenitor cells in diabetic wound healing. Cell Transplant 19:1369–1381
Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA (2007) Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109:1801–1809
Herdrich BJ, Lind RC, Liechty KW (2008) Multipotent adult progenitor cells: their role in wound healing and the treatment of dermal wounds. Cytotherapy 10:543–550
Liu SH, Sheu WH, Lee MR, Lee WJ, Yi YC, Yang TJ, Jen JF, Pan HC, Shen CC, Chen WB, Tien HR, Sheu ML (2013) Advanced glycation end product Nepsilon-carboxymethyllysine induces endothelial cell injury: the involvement of SHP-1-regulated VEGFR-2 dephosphorylation. J Pathol 230:215–227
Portero-Otin M, Pamplona R, Bellmunt MJ, Ruiz MC, Prat J, Salvayre R, Negre-Salvayre A (2002) Advanced glycation end product precursors impair epidermal growth factor receptor signaling. Diabetes 51:1535–1542
Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB (2004) Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 24:288–293
Lois N, McCarter RV, O’Neill C, Medina RJ, Stitt AW (2014) Endothelial progenitor cells in diabetic retinopathy. Front Endocrinol (Lausanne) 5:44
Ilan N, Mahooti S, Madri JA (1998) Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J Cell Sci 111(Pt 24):3621–3631
Pepper MS, Vassalli JD, Orci L, Montesano R (1993) Biphasic effect of transforming growth factor-beta 1 on in vitro angiogenesis. Exp Cell Res 204:356–363
Lindstedt KA, Wang Y, Shiota N, Saarinen J, Hyytiainen M, Kokkonen JO, Keski-Oja J, Kovanen PT (2001) Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J 15:1377–1388
Galli SJ, Tsai M (2008) Mast cells: versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J Dermatol Sci 49:7–19
Iba Y, Shibata A, Kato M, Masukawa T (2004) Possible involvement of mast cells in collagen remodeling in the late phase of cutaneous wound healing in mice. Int Immunopharmacol 4:1873–1880
Bevan D, Gherardi E, Fan TP, Edwards D, Warn R (2004) Diverse and potent activities of HGF/SF in skin wound repair. J Pathol 203:831–838
Kitamura Y, Go S, Hatanaka K (1978) Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood 52:447–452
Okayama Y, Kawakami T (2006) Development, migration, and survival of mast cells. Immunol Res 34:97–115
Ashman LK (1999) The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol 31:1037–1051
Galli SJ, Tsai M, Wershil BK (1993) The c-kit receptor, stem cell factor, and mast cells. What each is teaching us about the others. Am J Pathol 142:965–974
Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, Geissler EN, Galli SJ (1991) Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci USA 88:6382–6386
Dudeck A, Leist M, Rubant S, Zimmermann A, Dudeck J, Boehncke WH, Maurer M (2010) Immature mast cells exhibit rolling and adhesion to endothelial cells and subsequent diapedesis triggered by E- and P-selectin, VCAM-1 and PECAM-1. Exp Dermatol 19:424-434
Zins SR, Amare MF, Tadaki DK, Elster EA, Davis TA (2010) Comparative analysis of angiogenic gene expression in normal and impaired wound healing in diabetic mice: effects of extracorporeal shock wave therapy. Angiogenesis 13:293–304
Karimi K, Redegeld FA, Heijdra B, Nijkamp FP (1999) Stem cell factor and interleukin-4 induce murine bone marrow cells to develop into mast cells with connective tissue type characteristics in vitro. Exp Hematol 27:654–662
Cavalher-Machado SC, de Lima WT, Damazo AS, de Frias Carvalho V, Martins MA, e Silva PM, Sannomiya P (2004) Down-regulation of mast cell activation and airway reactivity in diabetic rats: role of insulin. Eur Respir J 24:552–558
Wu L, Derynck R (2009) Essential role of TGF-beta signaling in glucose-induced cell hypertrophy. Dev Cell 17:35–48
Lee MJ, Kim J, Lee KI, Shin JM, Chae JI, Chung HM (2011) Enhancement of wound healing by secretory factors of endothelial precursor cells derived from human embryonic stem cells. Cytotherapy 13:165–178
Broadley KN, Aquino AM, Hicks B, Ditesheim JA, McGee GS, Demetriou AA, Woodward SC, Davidson JM (1989) The diabetic rat as an impaired wound healing model: stimulatory effects of transforming growth factor-beta and basic fibroblast growth factor. Biotechnol Ther 1:55–68
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishikori, Y., Shiota, N. & Okunishi, H. The role of mast cells in cutaneous wound healing in streptozotocin-induced diabetic mice. Arch Dermatol Res 306, 823–835 (2014). https://doi.org/10.1007/s00403-014-1496-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-014-1496-0