Abstract
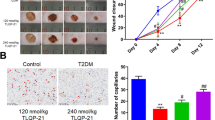
Non-healing diabetic wounds (DW) are a serious clinical problem that remained poorly understood. We recently found that topical application of growth differentiation factor 11 (GDF11) accelerated skin wound healing in both Type 1 DM (T1DM) and genetically engineered Type 2 diabetic db/db (T2DM) mice. In the present study, we elucidated the cellular and molecular mechanisms underlying the action of GDF11 on healing of small skin wound. Single round-shape full-thickness wound of 5-mm diameter with muscle and bone exposed was made on mouse dorsum using a sterile punch biopsy 7 days following the onset of DM. Recombinant human GDF11 (rGDF11, 50 ng/mL, 10 μL) was topically applied onto the wound area twice a day until epidermal closure (maximum 14 days). Digital images of wound were obtained once a day from D0 to D14 post-wounding. We showed that topical application of GDF11 accelerated the healing of full-thickness skin wounds in both type 1 and type 2 diabetic mice, even after GDF8 (a muscle growth factor) had been silenced. At the cellular level, GDF11 significantly facilitated neovascularization to enhance regeneration of skin tissues by stimulating mobilization, migration and homing of endothelial progenitor cells (EPCs) to the wounded area. At the molecular level, GDF11 greatly increased HIF-1ɑ expression to enhance the activities of VEGF and SDF-1ɑ, thereby neovascularization. We found that endogenous GDF11 level was robustly decreased in skin tissue of diabetic wounds. The specific antibody against GDF11 or silence of GDF11 by siRNA in healthy mice mimicked the non-healing property of diabetic wound. Thus, we demonstrate that GDF11 promotes diabetic wound healing via stimulating endothelial progenitor cells mobilization and neovascularization mediated by HIF-1ɑ-VEGF/SDF-1ɑ pathway. Our results support the potential of GDF11 as a therapeutic agent for non-healing DW.








Similar content being viewed by others
References
Roop D. Defects in the barrier. Science. 1995;267:474–5.
Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol. 2009;4:71–95.
Watt SM, Pleat JM. Stem cells, niches and scaffolds: applications to burns and wound care. Adv Drug Deliv Rev. 2018;123:82–106. 1
Wukich DK. Diabetes and its negative impact on outcomes in orthopaedic surgery. World J Orthop. 2015;6:331–9.
Wukich DK, Raspovic KM, Suder NC. Patients with diabetic foot disease fear major lower-extremity amputation more than death. Foot Ankle Spec. 2017:1938640017694722.
Robbins JM, Strauss G, Aron D, Long J, Kuba J, et al. Mortality rates and diabetic foot ulcers: is it time to communicate mortality risk to patients with diabetic foot ulceration? J Am Podiatr Med Assoc. 2008;98:489–93.
Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367–75.
Shen Y, Guo Y, Mikus P, Sulniute R, Wilczynska M, Ny T, et al. Plasminogen is a key proinflammatory regulator that accelerates the healing of acute and diabetic wounds. Blood. 2012;119:5879–87.
Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med. 2011;3:100ra89.
Kronemann N, Bouloumi A, Bassus S, Kirchmaier CM, Busse R, Schini-Kerth VB. Aggregating human platelets stimulate expression of vascular endothelial growth factor in cultured vascular smooth muscle cells through a synergistic effect of transforming growth factor-beta 1 and platelet-derived growth factor (AB). Circulation. 1999;100:855–60.
Sunderkotter C, Goebeler M, Schulze-Osthoff K, Bhardwaj R, Sorg C. Macrophage-derived neovascularization factors. Pharmacol Ther. 1991;51:195–216.
De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour neovascularization. Nat Rev Cancer. 2017;17:457–74.
Bose D, Meric-Bernstam F, Hofstetter W, Reardon DA, Flaherty KT, Ellis LM. Vascular endothelial growth factor targeted therapy in the perioperative setting: implications for patient care. Lancet Oncol. 2010;11:373–82.
Kundra V, Escobedo JA, Kazlauskas A, Kim HK, Rhee SG, Williams LT, et al. Regulation of chemotaxis by the platelet-derived growth factor receptor-beta. Nature. 1994;367:474–6.
Robson MC, Phillips LG, Thomason A, Robson LE, Pierce GF. Platelet-derived growth factor BB for the treatment of chronic pressure ulcers. Lancet. 1992;339:23–25.
Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–4.
Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–39.
Leinwand LA, Harrison BC. Young at heart. Cell. 2013;153:743–5.
Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22:164–74.
Smith SC, Zhang X, Zhang X, Gross P, Starosta T, Mohsin S, et al. GDF11 does not rescue aging-related pathological hypertrophy. Circ Res. 2015;117:926–32.
Poggioli T, Vujic A, Yang P, Macias-Trevino C, Uygur A, Loffredo FS, et al. Circulating growth differentiation factor 11/8 levels decline with age. Circ Res. 2016;118:29–37.
Du GQ, Shao ZB, Wu J, Yin WJ, Li SH, Wu J, et al. Targeted myocardial delivery of GDF11 gene rejuvenates the aged mouse heart and enhances myocardial regeneration after ischemia-reperfusion injury. Basic Res Cardiol. 2017;112:7.
Finkenzeller G, Stark GB, Strassburg S. Growth differentiation factor 11 supports migration and sprouting of endothelial progenitor cells. J Surg Res. 2015;198:50–6.
Boucher JM, Clark RP, Chong DC, Citrin KM, Wylie LA, Bautch VL. Dynamic alterations in decoy VEGF receptor-1 stability regulate neovascularization. Nat Commun. 2017;8:15699.
Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6.
Gawaz M, Vogel S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood. 2013;122:2550–4.
Li Q, Jiao L, Shao Y, Li M, Gong M, Zhang Y, et al. Topical GDF11 accelerates skin wound healing in both type 1 and 2 diabetic mouse models. Biochem Biophys Res Commun. 2020;529:7–14.
Guo W, Feng JM, Yao L, Sun L, Zhu GQ. Transplantation of endothelial progenitor cells in treating rats with IgA nephropathy. BMC Nephrol. 2014;15:110.
Yi DA, Thomas EU, Alexandra G, Amy J, Alla D. Angiogenic potential of cryopreserved amniotic membrane is enhanced through retention of all tissue components in their native state. Adv Wound Care. 2015;4:513–22.
Zhang YH, Cheng F, Du XT, Gao JL, Xiao XL, Li N, et al. GDF11/BMP11 activates both smad1/5/8 and smad2/3 signals but shows no significant effect on proliferation and migration of human umbilical vein endothelial cells. Oncotarget. 2016;7:12063–74.
Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–84.
Belting M, Dorrell MI, Sandgren S, Aguilar E, Ahamed J, Dorfleutner A, et al. Regulation of neovascularization by tissue factor cytoplasmic domain signaling. Nat Med. 2004;10:502–9.
Patan S. Vasculogenesis and angiogenesis. Cancer Treat Res. 2004;117:3–32.
Rana D, Kumar A, Sharma S. Endothelial progenitor cells as molecular targets in vascular senescence and repair. Curr Stem Cell Res Ther. 2018;13:438–46.
Aday S, Zoldan J, Besnier M, Carreto L, Saif J, Fernandes R, et al. Synthetic microparticles conjugated with VEGF165 improve the survival of endothelial progenitor cells via microRNA-17 inhibition. Nat Commun. 2017;8:747.
Sawada N, Jiang A, Takizawa F, Safdar A, Manika A, Tesmenitsky Y, et al. Endothelial PGC-1alpha mediates vascular dysfunction in diabetes. Cell Metab. 2014;19:246–58.
Lerman OZ, Greives MR, Singh SP, Thanik VD, Chang CC, Seiser N, et al. Low-dose radiation augments vasculogenesis signaling through HIF-1-dependent and -independent SDF-1 induction. Blood. 2010;116:3669–76.
Kaur S, Tripathi D, Dongre K, Garg V, Rooge S, Mukopadhyay A, et al. Increased number and function of endothelial progenitor cells stimulate neovascularization by resident liver sinusoidal endothelial cells (SECs) in cirrhosis through paracrine factors. J Hepatol. 2012;57:1193–8.
Krishnamurthy P, Thal M, Verma S, Hoxha E, Lambers E, Ramirez V, et al. Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circ Res. 2011;109:1280–9.
Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, de Kreutzenberg S, et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care. 2010;33:1607–9.
Harmon EB, Apelqvist AA, Smart NG, Gu X, Osborne DH, Kim SK. GDF11 modulates NGN3+ islet progenitor cell number and promotes beta-cell differentiation in pancreas development. Development. 2004;131:6163–74.
Li H, Li Y, Xiang L, Zhang J, Zhu B, Xiang L, et al. GDF11 attenuates development of type 2 diabetes via improvement of islet beta-cell function and survival. Diabetes. 2017;66:1914–27.
Rezende F, Moll F, Walter M, Helfinger V, Hahner F, Janetzko P, et al. The NADPH organizers NoxO1 and p47phox are both mediators of diabetes-induced vascular dysfunction in mice. Redox Biol. 2017;15:12–21.
Safar ME. Arterial stiffness as a risk factor for clinical hypertension. Nat Rev Cardiol. 2018;15:97–105.
Tanaka R, Vaynrub M, Masuda H, Ito R, Kobori M, Miyasaka M, et al. Quality-control culture system restores diabetic endothelial progenitor cell vasculogenesis and accelerates wound closure. Diabetes. 2013;62:3207–17.
Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–59.
Takeda N, Maemura K, Imai Y, Harada T, Kawanami D, Nojiri T, et al. Endothelial PAS domain protein 1 gene promotes neovascularization through the transactivation of both vascular endothelial growth factor and its receptor, Flt-1. Circ Res. 2004;95:146–53.
Chen L, Endler A, Uchida K, Horiguchi S, Morizane Y, Iijima O, et al. Int6/eIF3e silencing promotes functional blood vessel outgrowth and enhances wound healing by upregulating hypoxia-induced factor 2 alpha expression. Circulation. 2010;122:910–9.
Ozawa K, Kondo T, Hori O, Kitao Y, Stern DM, Eisenmenger W, et al. Expression of the oxygen-regulated protein ORP150 accelerates wound healing by modulating intracellular VEGF transport. J Clin Invest. 2001;108:41–50.
Yang Y, Huang K, Wang M, Wang Q, Chang H, Liang Y, et al. Ubiquitination flow repressors: enhancing wound healing of infectious diabetic ulcers through stabilization of polyubiquitinated hypoxia-inducible factor-1α by theranostic nitric oxide nanogenerators. Adv Mater. 2021;33:e2103593.
Ii M, Takeshita K, Ibusuki K, Luedemann C, Wecker A, Eaton E, et al. Notch signaling regulates endothelial progenitor cell activity during recovery from arterial injury in hypercholesterolemic mice. Circulation. 2010;121:1104–12.
Dai X, Yan X, Zeng J, Chen J, Wang Y, Chen J, et al. Elevating CXCR7 improves angiogenic function of EPCs via Akt/GSK-3beta/Fyn-mediated Nrf2 activation in diabetic limb ischemia. Circ Res. 2017;120:e7–e23.
Li FY, Lam KS, Tse HF, Chen C, Wang Y, Vanhoutte PM, et al. Endothelium-selective activation of AMP-activated protein kinase prevents diabetes mellitus-induced impairment in vascular function and reendothelialization via induction of heme oxygenase-1 in mice. Circulation. 2012;126:1267–77.
Hiesinger W, Perez-Aguilar JM, Atluri P, Marotta NA, Frederick JR, Fitzpatrick JR, et al. Computational protein design to reengineer stromal cell-derived factor-1alpha generates an effective and translatable angiogenic polypeptide analog. Circulation. 2011;124:S18–26.
Climent M, Quintavalle M, Miragoli M, Chen J, Condorelli G, Elia L. TGFβ triggers mir-143/145 transfer from smooth muscle cells to endothelial cells, thereby modulating vessel stabilization. Circ Res. 2015;116:1753–64.
Wang W, Qu R, Wang X, Zhang M, Zhang Y, Chen C, et al. GDF11 antagonizes psoriasis-like skin inflammation via suppression of NF-κB signaling pathway. Inflammation. 2019;42:319–30.
Kanitkar M, Jaiswal A, Deshpande R, Bellare J, Kale VP. Enhanced growth of endothelial precursor cells on PCG-matrix facilitates accelerated, fibrosis-free, wound healing: a diabetic mouse model. PLoS One. 2013;8:e69960.
Nishimura Y, Ii M, Qin G, Hamada H, Asai J, Takenaka H, et al. CXCR4 antagonist AMD3100 accelerates impaired wound healing in diabetic mice. J Invest Dermatol. 2012;132:711–20.
Acknowledgements
This work was supported in part by the grants from National Key R&D Program of China (2017YFC1307403), the National Natural Science Foundation of China (81730012, 91949130, 81970320, 82003749 and 81970202), and The National Key Research and Development Program of China–Traditional Chinese Medicine Modernization Research project 2017YFC1702000 (2017YFC1702003). Natural Science Foundation of Heilongjiang province (LC2018034).
Author information
Authors and Affiliations
Contributions
Ying Zhang and YYZ conceived and designed all experiments. QQL, XWY, YYW, and HDL conducted diabetic wound healing model. MYZ, DHL, YYZ and ZWP identified the EPCs function in vivo and in vitro. YYZ, QQL, LJ, LHS conducted MicroPET/CT. YYZ, LNX, YCS, MML, MYG, XFZ, YMZ, ZGL, ZYT, YYZ, QY, and YQL performed all of the other experiments in this study. YYZ, Ying Zhang, LJ, XL, Yong Zhang and BFY discussed the data and wrote this paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Zhang, Yy., Pan, Zw. et al. GDF11 promotes wound healing in diabetic mice via stimulating HIF-1ɑ-VEGF/SDF-1ɑ-mediated endothelial progenitor cell mobilization and neovascularization. Acta Pharmacol Sin 44, 999–1013 (2023). https://doi.org/10.1038/s41401-022-01013-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-01013-2
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Ang-1 and VEGF: central regulators of angiogenesis
Molecular and Cellular Biochemistry (2024)
-
Panax notoginseng Saponins Play a Protective Role in Acute Cerebral Infarction by Regulating lncRNA SNHG15
Revista Brasileira de Farmacognosia (2023)