Abstract
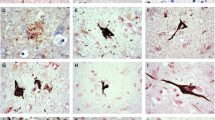
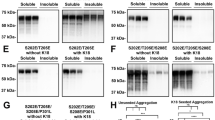
Microtubule-associated protein tau is the major component of the filamentous neurofibrillary lesions of Alzheimer’s disease (AD) and other tauopathies. Recently, it has been reported that tau isoforms lacking both N-terminal exon 2 and exon 3 do not form straight filament- or paired helical filament-like filaments in vitro, and that the N-terminal exons facilitate assembly of full-length tau. However, neuropathological and biological studies on the N-terminal region of tau protein in human tissue have been limited. We performed a biochemical study on the abnormally phosphorylated tau in brains affected by AD and corticobasal degeneration (CBD), and an immunohistochemical study on tau-positive structures in neurodegenerative diseases, to clarify whether tau with the exon 3 insert was present in abnormal tau-positive structures. On immunoblots of sarkosyl-insoluble tau, anti-exon 3 antibody (anti-E3 Ab) recognized two bands of 68 and 72 kDa in AD and only one band of 72 kDa in CBD. Immunohistochemically, anti-E3 Ab recognized most parts of the neurofibrillary tangles (NFT) in AD and Pick bodies in Pick’s disease. In progressive supranuclear palsy (PSP) and CBD, most NFT and pretangles were positive for anti-E3 Ab, as were a small number of glial inclusions. These results indicate that abnormally phosphorylated tau containing the exon 3 insert is present in both PSP and CBD brain, and that CBD cannot be distinguished from PSP by immunoreactivity for anti-E3 Ab. Although most intraglial inclusions were negative for anti-E3 Ab, a few were positive. Therefore, tau isoforms containing the exon 3 insert are expressed at low levels in glial cells.




Similar content being viewed by others
References
Brandt R, Leger J, Lee G (1995) Interaction of tau with the neural plasma membrane mediated by tau’s amino-terminal projection domain. J Cell Biol 131:1327–1340
Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33:95–130
Buee-Scherrer V, Hof PR, Buee L, Leveugle B, Vermersch P, Perl DP, Olanow CW, Delacourte A (1996) Hyperphosphorylated tau proteins differentiate corticobasal degeneration and Pick’s disease. Acta Neuropathol 91:351–359
Chen J, Kanai Y, Cowan NJ, Hirokawa N (1992) Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature 360:674–677
Delacourte A, Sergeant N, Wattez A, Gauvreau D, Robitaille Y (1998) Vulnerable neuronal subsets in Alzheimer’s and Pick’s disease are distinguished by their tau isoform distribution and phosphorylation. Ann Neurol 43:193–204
Feany MB, Ksiezak-Reding H, Liu WK, Vincent I, Yen SH, Dickson DW (1995) Epitope expression and hyperphosphorylation of tau protein in corticobasal degeneration: differentiation from progressive supranuclear palsy. Acta Neuropathol 90:37–43
Flament S, Delacourte A, Verny M, Hauw JJ, Javoy-Agid F (1991) Abnormal Tau proteins in progressive supranuclear palsy. Similarities and differences with the neurofibrillary degeneration of the Alzheimer type. Acta Neuropathol 81:591–596
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3:519–526
Goedert M, Spillantini MG, Cairns NJ, Crowther RA (1992) Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 8:159–168
Hirokawa N, Shiomura Y, Okabe S (1988) Tau proteins: the molecular structure and mode of binding on microtubules. J Cell Biol 107:1449–1459
Ikeda K, Akiyama H, Arai T, Matsushita M, Niizato K, Kuroki N, Iritani S, Tsuchiya K (1998) Argyrophilic grain dementia (Braak)—Clinicopathological evaluation of three cases (in Japanese). Shinkei Kenkyu no Shinpo 42:855–866
Ishiguro K, Sato K, Takamatsu M, Park JM, Uchida T, Imahori K (1995) Analysis of phosphorylation of tau with antibodies specific for phosphorylation sites. Neurosci Lett 202:81–84
King ME, Gamblin TC, Kuret J, Binder LI (2000) Differential assembly of human tau isoforms in the presence of arachidonic acid. J Neurochem 74:1749–1757
Ksiezak-Reding H, Binder LI, Yen SH (1990) Alzheimer disease proteins (A68) share epitopes with tau but show distinct biochemical properties. J Neurosci Res 25:420–430
Ksiezak-Reding H, Morgan K, Mattiace LA, Davies P, Liu WK, Yen SH, Weidenheim K, Dickson DW (1994) Ultrastructure and biochemical composition of paired helical filaments in corticobasal degeneration. Am J Pathol 145:1496–1508
Nishimura T, Ikeda K, Akiyama H, Arai T, Kondo H, Okochi M, Furiya Y, Mori H, Oda T, Kato M, Iseki E (1997) Glial tau-positive structures lack the sequence encoded by exon 3 of the tau protein gene. Neurosci Lett 224:169–172
Sergeant N, David JP, Goedert M, Jakes R, Vermersch P, Buee L, Lefranc D, Wattez A, Delacourte A (1997) Two-dimensional characterization of paired helical filament-tau from Alzheimer’s disease: demonstration of an additional 74-kDa component and age-related biochemical modifications. J Neurochem 69:834–844
Sergeant N, Wattez A, Delacourte A (1999) Neurofibrillary degeneration in progressive supranuclear palsy and corticobasal degeneration: tau pathologies with exclusively “exon 10” isoforms. J Neurochem 72:1243-1249
Spillantini MG, Goedert M, Crowther RA, Murrell JR, Farlow MR, Ghetti B (1997) Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci USA 94:4113–4118
Vermersch P, Robitaille Y, Bernier L, Wattez A, Gauvreau D, Delacourte A (1994) Biochemical mapping of neurofibrillary degeneration in a case of progressive supranuclear palsy: evidence for general cortical involvement. Acta Neuropathol 87:572–577
Yanagawa H, Chung SH, Ogawa Y, Sato K, Shibata-Seki T, Masai J, Ishiguro K (1998) Protein anatomy: C-tail region of human tau protein as a crucial structural element in Alzheimer’s paired helical filament formation in vitro. Biochemistry 37:1979–1988
Acknowledgements
The authors thank Ms. M. Onbe and Ms. A Kajitani for their skillful technical assistance. This study was partly supported by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (15591227), and a research grant from Zikei Institute of Psychiatry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Terada, S., Ishizu, H., Ishiguro, K. et al. Exon 3 insert of tau protein in neurodegenerative diseases. Acta Neuropathol 110, 12–18 (2005). https://doi.org/10.1007/s00401-005-1012-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-005-1012-x