Abstract
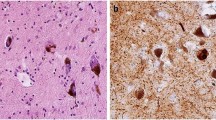
Our institution is currently engaged in ongoing genetic studies of familial Alzheimer's disease (AD), which include clinical ascertainment and brain autopsy of both affected and non-affected family members. Here we describe the analysis of 22 AD families, each with at least one family member with a postmortem diagnosis of dementia with Lewy bodies (DLB). For this study, 47 brains were examined according to NINCDS-Reagan Institute criteria for the diagnosis of AD. Lewy body pathology was evaluated with α-synuclein immunohistochemistry. Four families, with either one or two autopsies showing Lewy body pathology, demonstrated linkage to 12p. Five families had two or more autopsies with Lewy body pathology, but their linkage status was unknown. The remaining 13 families had one autopsy demonstrating Lewy bodies. These findings suggest that at least one pathological form of DLB may be familial. In some families, the pathological phenotype is identical in all examined affected family members; but in others, there may be several pathologies that coexist. Careful neuropathological examination of affected family members may prove critical for future genetic analysis of AD and DLB.

Similar content being viewed by others
References
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259
Dickson DW, Crystal H, Mattiace LA, et al (1989) Diffuse Lewy body disease: light and electron microscopic immunocytochemistry of senile plaques. Acta Neuropathol 78:572–584
Galasko D, Hansen LA, Katzman R, et al (1994) Clinical-neuropathological correlations in Alzheimer's disease and related dementias. Arch Neurol 51:888–895
Hashimoto M, Hsu LJ, Rockenstein E, Takenouchi T, Mallory M, Masliah E (2002) α-Synuclein protects against oxidative stress via inactivation of the C-jun N-terminal kinase stress signaling pathway in neuronal cells. J Biol Chem 14:14
Hsu LJ, Sagara Y, Arroyo A, et al (2000) Alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol 157:401–410
Hulette C, Mirra S, Wilkinson W, Heyman A, Fillenbaum G, Clark C (1995) The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part IX. A prospective cliniconeuropathologic study of Parkinson's features in Alzheimer's disease. Neurology 45:1991–1995
Hulette CM, Welsh-Bohmer KA, Crain B, Szymanski MH, Sinclaire NO, Roses AD (1997) Rapid brain autopsy. The Joseph and Kathleen Bryan Alzheimer's Disease Research Center experience. Arch Pathol Lab Med 121:615–618
Hyman BT, Trojanowski JQ (1997) Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 56:1095–1097
Ishikawa A, Takahashi H, Tanaka H, Hayashi T, Tsuji S (1997) Clinical features of familial diffuse Lewy body disease. Eur Neurol 38:34–38
Kahle PJ, Neumann M, Ozmen L, et al (2000) Subcellular localization of wild-type and Parkinson's disease-associated mutant alpha -synuclein in human and transgenic mouse brain. J Neurosci 20:6365–6373
Kanda S, Bishop JF, Eglitis MA, Yang Y, Mouradian MM (2000) Enhanced vulnerability to oxidative stress by alpha-synuclein mutations and C-terminal truncation. Neuroscience 97:279–284
Kehoe P, Wavrant-De Vrieze F, Crook R, et al (1999) A full genome scan for late onset Alzheimer's disease. Hum Mol Genet 8:237–245
Lippa CF, Fujiwara H, Mann DM, et al (1998) Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer's disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 153:1365–1370
Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ (1999) Antibodies to alpha-synuclein detect Lewy bodies in many Down's syndrome brains with Alzheimer's disease. Ann Neurol 45:353–357
Masliah E, Rockenstein E, Veinbergs I, et al (2000) Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287:1265–1269
Masliah E, Rockenstein E, Veinbergs I, et al (2001) Beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc Natl Acad Sci USA 98:12245–12250
McKeith IG, Galasko D, Kosaka K, et al (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 47:1113–1124
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E (1984) Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34:939–944
Mirra SS, Heyman A, McKeel D, et al (1991) The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathological assessment of Alzheimer's disease. Neurology 41:479–486
Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B (2000) The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci 20:6048–6054
Pericak-Vance, MA, Bass MP, Yamaoka LH, et al (1997) Complete genomic screen in late-onset familial Alzheimer disease. Evidence for a new locus on chromosome 12. JAMA 278:1237–1241
Rogaeva E, Premkumar S, Song Y, et al (1998) Evidence for an Alzheimer disease susceptibility locus on chromosome 12 and for further locus heterogeneity. JAMA 280:614–618
Rosenberg CK, Pericak-Vance MA, Saunders AM, Gilbert JR, Gaskell PC, Hulette CM (2000) Lewy body and Alzheimer pathology in a family with the amyloid-beta precursor protein APP717 gene mutation. Acta Neuropathol 100:145–152
Rosenberg CK, Cummings TJ, Saunders AM, Widico C, McIntyre LM, Hulette CM (2001) Dementia with Lewy bodies and Alzheimer's disease. Acta Neuropathol 102:621–626
Saha AR, Ninkina NN, Hanger DP, Anderton BH, Davies AM, Buchman VL (2000) Induction of neuronal death by alpha-synuclein. Eur J Neurosci 12:3073–3077
Scott WK, Grubber JM, Abou-Donia SM, et al (1999) Further evidence linking late-onset Alzheimer disease with chromosome 12. JAMA 281:513–514
Scott WK, Grubber JM, Conneally PM, et al (2000) Fine mapping of the chromosome 12 late-onset Alzheimer disease locus: potential genetic and phenotypic heterogeneity. Am J Hum Genet 66:922–932
Stefanova N, Klimaschewski L, Poewe W, Wenning GK, Reindl M (2001) Glial cell death induced by overexpression of alpha-synuclein. J Neurosci Res 65:432–438
Swanberg MM, Cummings JL (2002) Benefit-risk considerations in the treatment of dementia with Lewy bodies. Drug Saf 25:511–523
Uversky VN, Lie J, Fink AL (2001) Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem 276:10737–10744
Wu WS, Holmans P, Wavrant-DeVrieze F, et al (1998) Genetic studies on chromosome 12 in late-onset Alzheimer disease. JAMA 280:619–622
Zhou W, Hurlbert MS, Schaack J, Prasad KN, Freed CR (2000) Overexpression of human alpha-synuclein causes dopamine neuron death in rat primary culture and immortalized mesencephalon-derived cells. Brain Res 866:33–43
Acknowledgements
The authors gratefully acknowledge the assistance of Autopsy Nurse Coordinators, Mari Szymanski RN, C. and Nancy Sinclaire RN, C. now deceased. We also wish to thank Anne Latham for careful assistance with manuscript preparation. Supported by NIA AG05128; AG19757 and NINDS N39764, Glaxo SmithKline and numerous donations from families to the Joseph and Kathleen Price Bryan Alzheimer's Disease Research Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trembath, Y., Rosenberg, C., Ervin, J.F. et al. Lewy body pathology is a frequent co-pathology in familial Alzheimer's disease. Acta Neuropathol 105, 484–488 (2003). https://doi.org/10.1007/s00401-003-0670-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-003-0670-9