Abstract
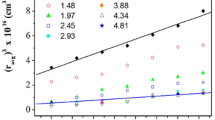
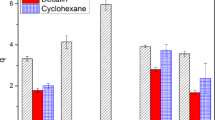
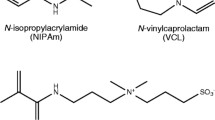
It has been known that zeta potential (ζ) or electrophoretic mobility of poly(N-isopropyl acrylamide) (PNIPAM) gel particles in water significantly increase in the magnitude with increasing the temperature. This phenomenon has been interpreted as caused by an enhancement of the surface charge density, which was derived from charged initiator used for the gel preparation, due to the temperature-induced gel deswelling. On the other hand, it has also been established that oil droplets dispersed in water show a negative ζ, which has been ascribed to a specific adsorption of OH− ions from aqueous phase to the hydrophobic hydration layer around the nonpolar droplets. In the present study, we attempted to see if the OH− adsorption actually contributes toζ of PNIPAM microgel particles prepared with four kinds of radical initiators including neutral one. ζ measurements were carried out to find that not only the neutral gel particles but also weakly cationic ones showed a significantly negativeζ at the higher temperature region where the gel collapse occurred. ζ values in the presence of 10−4 M NaCl, NaOH, or HCl were also measured and proved to be consistent with the OH− ion adsorption mechanism.








Similar content being viewed by others
References
Carruthers JC (1938) The electrophoresis of certain hydrocarbons and their derivatives as a function of pH. Trans Faraday Soc 34:300–307
Zimmermann R, Dukhin S, Werner C (2001) Electrokinetic measurements reveal interfacial charge at polymer films caused by simple electrolyte ions. J Phys Chem B 105:8544–8549
Takahashi M (2005) ζpotential of microbubbles in aqueous solutions: electrical properties of the gas-water interface. J Phys Chem B 109:21858–21864
Beattie JK, Djerdjev AM, Warr GG (2009) The surface of neat water is basic. Faraday Discuss 141:31–39
Marinova KG, Alargova ND, Denkov ND, Velev OD, Petsev DN, Ivanov IB, Borwankar RP (1996) Charging of oil-water interfaces due to spontaneous adsorption of hydroxyl ions. Langmuir 12:2045–2051
Gray-Weale A, Beattie JK (2009) An explanation for the charge on water’s surface. Phys Chem Chem Phys 11:10994–11005
Zangi R, Engberts JBFN (2005) Physisorption of hydroxide ions from aqueous solution to a hydrophobic surface. J Am Chem Soc 127:2272–2276
Vacha R, Zangi R, Engberts JBFN, Jungwirth P (2008) Water structuring and hydroxide ion binding at the interface between water and hydrophobic walls of varying rigidity and van der Waals interactions. J Phys Chem C 112:7689–7692
Schild HG (1992) Poly(N-isopropylacrylamide): experiment, theory and application. Prog Polym Sci 17:163–249
Zhang WZ, Chen XD, Luo W, Yang J, Zhang MQ, Zhu FM (2009) Study of phase separation of poly(vinyl methyl ether) aqueous solutions with Rayleigh scattering technique. Macromolecules 42:1720–1725
Pelton RH, Pelton HM, Morphesis A, Rowell RL (1989) Particle sizes and electrophoretic mobilities of poly(N-isopropylacrylamide) latex. Langmuir 5:816–818
Daly E, Saunders BR (2000) Temperature-dependent electrophoretic mobility and hydrodynamic radius measurements of poly(N-isopropylacrylamide) microgel particles: structural insights. Phys Chem Chem Phys 2:3187–3193
Wu X, Pelton RH, Hamielec AE, Woods DR, McPhee W (1994) The kinetics of poly(N-isopropylacrylamide) microgel latex formation. Colloid Polym Sci 272:467–477
Lopez-Leon T, Ortega-Vinuesa JL, Bastos-Gonzalez D, Elaissari A (2006) Cationic and anionic poly(N-isopropylacrylamide) based submicron gel particles: electrokinetic properties and colloidal stability. J Phys Chem B 110:4629–4636
Tauer K, Gau D, Schulze S, Völkel A, Dimova R (2009) Thermal property changes of poly(N-isopropylacrylamide) microgel particles and block copolymers. Colloid Polym Sci 287:299–312
Aseyev VO, Tenhu H, Winnik FM (2006) Temperature dependence of the colloidal stability of neutral amphiphilic polymers in water. Adv Polym Sci 196:1–85
Ohshima H (2007) Electrokinetics of soft particles. Colloid Polym Sci 285:1411–1421
Saunders BR, Vincent B (1999) Microgel particles as model colloids: theory, properties and applications. Adv Colloid Interf Sci 80:1–25
Bondareva SO, Murinov YI, Spirikhin SV (2000) Protonation of 1,2-disubstituted imidazolines and other products of condensation of 2-ethylhexanoic acid with diethylenetriamine and triethylenetetramine. Russ Chem Bull Int Ed 49:2041–2046
Clayden JP, Greeves N, Warren S, Wothers PD (2001) Organic chemistry, Oxford University Press, p. 202
Creux P, Lachaise J, Graciaa A, Beattie JK, Djerdjev AM (2009) Strong specific hydroxide ion binding at the pristine oil/water and air/water interfaces. J Phys Chem B 113:14146–14150
Iuchi S, Chen H, Paesani F, Voth GA (2009) Hydrated excess proton at water-hydrophobic interfaces. J Phys Chem B 113:4017–4030
Hou L, Wu P (2015) Comparison of LCST-transitions of homopolymer mixture, diblock and statistical copolymers of NIPAM and VCL in water. Soft Matter 11:2771–2781
Horne RA, Almeida JP, Day AF, Yu N-T (1971) Macromolecule hydration and the effect of solutes on the cloud point of aqueous solutions of polyvinyl methyl ether; a possible model for protein denaturation and temperature control in homeothermic animals. J Colloid Interf Sci 35:77–84
Tong Z, Zeng F, Zheng X, Sato T (1999) Inverse molecular weight dependence of cloud points for aqueous poly(N-isopropylacrylamide) solutions. Macromolecules 32:4488–4490
Laukkanen A, Wiedmer SK, Varjo S, Riekkola TH (2002) Stability and thermosensitive properties of various poly(N-vinylcaprolactam) microgels. Colloid Polym Sci 280:65–70
Acknowledgements
We thank Dr. S. Uchida (Tokyo Institute of Technology, Department of Organic and Polymeric Materials) for the generous donation of VA-060.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Utashiro, Y., Takiguchi, M. & Satoh, M. Zeta potential of PNIPAM microgel particles dispersed in water—effects of charged radical initiators vs. OH− ion adsorption. Colloid Polym Sci 295, 45–52 (2017). https://doi.org/10.1007/s00396-016-3976-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3976-6