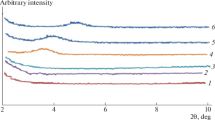
Abstract
Polystyrene (PS) colloid particles in presence of non-ionic surfactant-modified clay particles were prepared by the free-radical polymerization of styrene monomers in emulsion. Three different types of non-ionic surfactants, sorbitan monopalmitate (Span®40), polyethylene glycol octadecyl ether (Brij®S10), and polyoxyethylene (9) nonylphenylether (Igepal®Co-630) were used for the preparation of surfactant-modified clay. High-resolution transmission electron microscopy studies showed that few colloid PS particles with clay mineral layers at the surface were obtained; the particle sizes were observed to be in the micrometer size range, and stable dispersions were obtained when Span®40 and Igepal®Co-630 modified clay minerals were used as stabilizers. The clay mineral particles were observed to be mostly encapsulated by PS latex particles, and a typical morphology was observed when Brij®S10-modified clay was used as a stabilizer. This strategy can be applied to develop stable polymer latex particles via emulsion polymerization.

The formation of stable polystyrene colloidal particles using non-ionic surfactant modified clay mineral










Similar content being viewed by others
References
Anderson C, Daniels E (2003) Emulsion polymerization and latex applications. Rap Rev Rep 14:1–156
Asua JM (2004) Emulsion polymerization: from fundamental mechanisms to process developments. J Polym Sci Part A Polym Chem 42:1025–1041
Chern CS (2006) Emulsion polymerization mechanisms and kinetics. Prog Polym Sci 31:443–486
Asua J (2002) Miniemulsion polymerization. Prog Polym Sci 27:1283–1346
Schork F, Luo Y, Smulders W, Russum J, Butte A, Fontenot K (2005) Miniemulsion polymerization. Adv Polym Sci 175:129–255
Kawaguchi S, Ito K (2005) Dispersion polymerization. Adv Polym Sci 175:299–328
Saenz JM, Asua JM (1996) Dispersion polymerization of styrene and butylacrylate in polar solvents. J Polym Sci Part A Polym Chem 34:1977–1992
Wu Y, Zhang J, Zhao H (2008) Functional colloidal particles stabilized by layered silicate with hydrophilic face and hydrophobic polymer brushes. J Polym Sci Part A Polym Chem 47:1535–1543
Davis SS, Round HP, Purewal TS (1981) J Colloid Interface Sci 80:508–511
Webster AJ, Cates ME (1998) Stabilization of emulsions by trapped species. Langmuir 14:2068–2079
Jhaveri S, Koylu D, Maschke D, Carter K (2007) Synthesis of polymeric core-shell particles using surface-initiated living free-radical polymerization. J Polym Sci Part A Polym Chem 45:1575–1584
Abismail B, Canselier J, Wilhelm A, Delmas H, Gourdon C (1999) Emulsification by ultrasound: drop size distribution and stability. Ultrason Sonochem 6:75–83
Fontenot K, Schork F (1993) Sensitivities of droplet size and stability in monomeric emulsions. J Ind Eng Chem Res 32:373–385
Soma J, Papadopoulos K (1996) Ostwald ripening in sodium dodecyl sulfate-stabilized decane-in-water emulsions. J Colloid Interface Sci 181:225–231
Eshuis A, Leendertse H, Thoenes D (1991) Surfactant-free emulsion polymerization of styrene using crosslinked seed particles. Colloid Polym Sci 269:1086–1089
Usuki A, Kawasumi M, Kojima Y, Okada A, Kurauchi T, Kamigaito O (1993) Swelling behavior of montmorillonite cation exchanged for ω-amino acids by ɛ-caprolactam. J Mater Res 8:1174–1178
Bonnefond A, Paulis M, Bon S, Leiz J (2013) Surfactant-free miniemulsion polymerization of nBA/S stabilized by NaMMT: films with improved water resistance. Langmuir 29:2397–2405
Negrete-Herrera N, Putaux J, Bourgeat-Lami E (2006) Synthesis of polymer/laponite nanocomposite latex particles via emulsion polymerization using silylated and cation-exchange laponite clay platelets. Prog Solid State Chem 34:121–137
Greesh N, Sanderson R, Hartmann P (2012) Preparation of polystyrene-clay nanocomposites via dispersion polymerization using oligomeric styrene-montmorillonite as stabilizer. Polym Int 61:834–843
Voorn D, Ming W, Herk AV (2006) Polymer-clay nanocomposite latex particles by inverse Pickering emulsion polymerization stabilized with hydrophobic montmorillonite platelets. Macromolecules 39:2137–2143
Yang Y, Liu L, Zhang J, Li C, Zhao H (2007) PMMA colloid particles stabilized by layered silicate with PMMA-b-PDMAEMA block copolymer brushes. Langumir 23:2867–2873
Pickering SU (1907) Pickering emulsion. J Chem Soc Trans 91:2001–2021
Cauvin S, Colver P, Bon F (2005) Pickering stabilized miniemulsion polymerization: preparation of clay armored latexes. Macromolecules 38:7887–7889
Binks BP (2002) Particles as surfactants similarities and differences. Curr Opin Colloid Interface Sci 7:21–41
Guillota S, Bergayaa F, Azevedoa C, Warmonta F, Tranchant J (2009) Internally structured Pickering emulsions stabilized by clay mineral particles. J Colloid Interface Sci 333:563–569
Bon S, Colver P (2007) Pickering miniemulsion polymerization using laponite clay as a stabilizer. Langumir 23:8316–8322
Yang Y, Liu L, Zhang J, Li C, Zhao H (2007) PMMA colloid particles stabalization by layered silicate with PMMA-b-PDMAEMA block copolymer brushes. Langmuir 23:2867–2873
Zhang J, Chen K, Zhao H (2008) PMMA colloid particles armored by clay layers with PDMAEMA polymer brushes. J Polym Sci Part A Polym Chem 46:2632–2639
Putlitz B, Landfester K, Fischer H, Antonietti M (2001) The generation of armored latexes and hollow inorganic shells made of clay sheets by templating cationic miniemulsions and latexes. Adv Mater 13:500–504
Samakande A, Sanderson R, Hartmann P (2008) Encapsulated clay particles in polystyrene by RAFT mediated miniemulsion polymerization. J Polym Sci Part A Polym Chem 46:7114–7126
Voorn D, Ming W, Herk AV (2006) Clay platelets encapsulated inside latex particles. Macromolecules 39:4654–4656
Tiarks F, Landfester K, Antonietti M (2001) Silica nanoparticles as surfactants and fillers for latexe made by miniemulsion polymerizations. Langmuir 17:5775–5780
Negrete-Herrera N, Putaux L, David L, Bourgeat-Lami E (2006) Polymer/laponite composite colloids through emulsion polymerization: influence of the clay modification level on particle morphology. Macromolecules 39:9177–9184
Landfester K (2001) Polyreactions in miniemulsions. Macromol Rapid Commun 22:896–936
Klein, A. Daniels, E. Formulation components. In Emulsion polymerization and emulsion polymers. In Lovell, P.; El-Aasser, M., Eds. Eds. John Wiley & Sons Ltd: New York, 1997; pp 207–237
Binks BP, Lumsdon SO (1999) Stability of oil-in-water emulsions stabilised by silica particles. Phys Chem Chem Phys 1:3007–3016
Binks BP, Lumsdon SO (2000) Influence of particle wettability on the type and stability of surfactant-free emulsions. Langmuir 16:8622–8631
Taylor P (1998) Ostwald ripening in emulsion. Adv Colloid and Interface Sci 75:107–163
Welin-Berger K, Bergenstahl B (2000) Inhibition of Ostwald ripening in local anesthetic emulsions by using hydrophobic excipients in the disperse phase. Int J Pharm 200:249–260
Zeeb B, Gibis M, Fischer L, Weiss J (2012) Influence of interfacial properties on Ostwald ripening in crosslinked multilayered oil-in-water emulsions. J Colloid Interface Sci 387:65–73
Lin C, Wu J, Chern C (2013) Effects of the molecular weight of polymeric costabilizers on the Ostwald ripening behavior and the polymerization kinetics of styrene miniemulsions. Colloid Surf A Phys Chem Eng Asp 434:178–184
Voorhees PW (1985) The theory of Ostwald ripping. J Stat Phys 38:231–252
Kuehmann C, Voorhees P (1996) Ostwald ripening in ternary alloys. Metall Mater Trans A 27:937–943
Greenland D (1963) The adsorption of polyvinyl alcohols by montmorillonite. J Colloid Sci 18:647–664
Deng Y, Dixon JB, White GN (2006) Adsorption of polyacrylamide on smectite, illite, and kaolinite. Soil Sci Soc Amer J 70:297–304
Xi Y, Martens W, He H, Frost RL. Thermogravimetric analysis of organoclays intercalated with the surfactant octadecyltrimethylammonium bromide. J Therm Anal Calorim 81:91–97.
Tong Z, Deng Y (2008) Kinetics of miniemulsion polymerization of styrene in the presence of organoclays. Macromol Mater Eng 293:529–537
Vermaa S, Kumara S, Gokhaleb R, Burgess D (2011) Physical stability of nanosuspensions: investigation of the role of stabilizers on Ostwald ripening. Int J Pharm 406:145–152
Tcholakova S, Mitrinova Z, Golemanov K, Denkov N, Vethamuthu M, Ananthapadmanabhan K (2011) Control of Ostwald ripening by using surfactants with high surface modulus. Langmuir 27:14807–14819
Takahara K, Ikeda S, Ishino S, Tachi K, Ikeue K, Sakata T, Hasegawa T, Mori H, Matsurmura M, Ohtani B (2005) Asymmetrically modified silica particles: a simple particulate surfactant for stabilization of oil droplets in water. J Amer Chem Soc 127:6271–6281
Tong Z, Deng Y (2007) Synthesis of polystyrene encapsulated nanosaponite composite latex via miniemulsion polymerization. Polymer 48:4337–4343
Herrera NN, Letoffe JM, Putaux JL, David L, Lami EB (2004) Aqueous dispersions of silane-functionalized laponite clay platelets. A first step toward the elaboration of water-based polymer/clay nanocomposites. Langmuir 20:1564–1571
Huang X, Brittain WJ (2001) Synthesis and characterization of PMMA nanocomposites by suspension and emulsion polymerization. Macromolecules 34:3255–3260
Bouanani F, Bendedouch D, Hemeryb P, Bounaceur B (2008) Encapsulation of montmorillonite in nanoparticles by miniemulsion polymerization. Colloid Surf A Phys Chem Eng Asp 317:751–755
Acknowledgments
The authors would like to thank the CSIR, DST, and NRF, South Africa, for their financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
This section summarizes the physical properties of EFD clay and discussion on emulsion polymerization in presence of pure surfactants.(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Greesh, N., Ray, S.S. Impact of non-ionic surfactant chemical structure on morphology and stability of polystyrene nanocomposite latex. Colloid Polym Sci 294, 157–170 (2016). https://doi.org/10.1007/s00396-015-3743-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3743-0