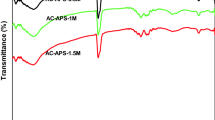
Abstract
Activated carbon and fullerene black react with cyclopentadiene at room temperature or slightly elevated temperature. At higher temperature a retro-Diels–Alder reaction takes place. The reaction with the diene and the retro-Diels–Alder reaction could be repeated. As a consequence of the reaction with cyclopentadiene or other suitable dienes and the retro reaction, the surface structure of different carbons changed considerably. The surface area of micropores on fullerene black was much higher than for the original sample. The influence on the surface area of porosity is reported for two different types of carbon.






Similar content being viewed by others
References
Beck MT, Szépvölgyi J, Szabó P, Jakab E (2001) Carbon 39:147
Hirsch A (1994) Chemistry of the fullerenes. Thieme, Stuttgart
Meidine MF, Roers R, Langley GJ, Avent AG, Darwish AD, Firth S, Kroto HW, Taylor R, Walton RK (1993) J Chem Soc Chem Commun 1342
Rubin Y, Khan S, Freedberg DI, Yeretzian C (1993) J Am Chem Soc 115:344
Tsuda M, Ishida T, Nogami T, Kurono S, Ohashi M (1993) J Chem Soc Chem Commun 1296
Gregg SJ, Sing KSW (1982) Adsorption, surface area and porosity. Academic, London
Glatter O, Kratky O (1982) In: Glatter O, Kratky O (eds) Small angle X-ray scattering. Academic, London, chap 2
Jánosi A, Stoeckli HF (1979) Carbon 17:465
Guinier A, Fournet G (1955) Small angle scattering of X-rays. Wiley, New York
Shull CG, Roess LC (1947) J Appl Phys 18:295
Bóta A, László K, Schlimper H, Nagy LG (1997) ACH Models Chem 134:169
Ko TH (1991) J Appl Polym Sci 43:589
Bóta A, László K, Nagy LG, Copitzky T (1997) Langmuir 13:6502
László K, Bóta A, Nagy LG (2000) Carbon 38:1965
Acknowledgement
The authors wish to thank the Hungarian National Scientific Fund OTKA T02522 T033004 and M36688 for scientific support.
Author information
Authors and Affiliations
Corresponding author
Appendix: SAXS by disperse porous systems
Appendix: SAXS by disperse porous systems
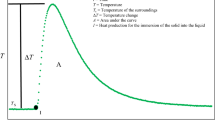
When X-rays are scattered by colloidal particles owing to differences in electron density caused by inhomogeneities, the intensity of scattered radiation is a function of the scattering vector h=4π/λsinΘ [7, 8, 9]:
where V is the volume of the system in which X-rays are scattered by electrons. η 2(0) is defined by [7, 8, 9]
where ρ e(r) is the local electron density at a given point r and ρ e is the average electron density.
The correlation function contains significant information on the geometry and structural arrangement of the scattering particles. The function is calculated from the scattering function [7, 10]. The following relationship holds for the tailing region of the scattering function (the so-called Porod range) [7]:
where S is the surface area of the particles. The specific surface area of the particles (relative to unit volume V) is [7]
where K p is the tail-end constant.
The correlation length is calculated directly from the scattering function by the following integration [7, 8, 9]:
From the middle section of the scattering curve the mass fractal dimension D m of the particles can be determined [10, 11], when the scattering curve is linear in a relatively wide range of h:
From the value of the tangent p, D m is calculated by the relationship D m=∣p∣+1 (for 1≤D m≤3).
Rights and permissions
About this article
Cite this article
T. Beck, M., Mándy, G., Papp, S. et al. Surface modification of activated carbon and fullerene black by Diels–Alder reaction. Colloid Polym Sci 283, 237–242 (2004). https://doi.org/10.1007/s00396-004-1139-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1139-7