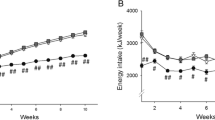
Abstract
Purpose
To assess hepatic de novo lipogenesis and mitochondrial energetics as well as whole-body energy homeostasis in sedentary rats fed a fructose-rich diet.
Methods
Male rats of 90 days of age were fed a high-fructose or control diet for 8 weeks. Body composition, energy balance, oxygen consumption, carbon dioxide production, non-protein respiratory quotient, de novo lipogenesis and insulin resistance were measured. Determination of specific activity of hepatic enzymes of de novo lipogenesis, mitochondrial mass, oxidative capacity and degree of coupling, together with parameters of oxidative stress and antioxidant defence, was also carried out.
Results
Body energy and lipid content as well as plasma insulin and non-esterified fatty acids were significantly higher in fructose-fed than in control rats. Significantly higher rates of net de novo lipogenesis and activities of hepatic lipogenic enzymes fatty acid synthase and stearoyl CoA desaturase-1 were found in fructose-fed rats compared to controls. Mitochondrial protein mass and degree of coupling were significantly higher in fructose-fed rats compared to controls. Hepatic mitochondria showed oxidative damage, both in the lipid and in the protein component, together with decreased activity of antioxidant defence.
Conclusion
Liver mitochondrial compartment is highly affected by fructose feeding. The increased mitochondrial efficiency allows liver cells to burn less substrates to produce ATP for de novo lipogenesis and gluconeogenesis. In addition, increased lipogenesis gives rise to whole body and ectopic lipid deposition, and higher mitochondrial coupling causes mitochondrial oxidative stress.



Similar content being viewed by others
References
Bray GA (2010) Soft drink consumption and obesity: it is all about fructose. Curr Opin Lipidol 21:51–57
Tappy L, Lê KA (2010) Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 90:23–46
Samuel VT (2011) Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metab 22:60–65
Brown CM, Dulloo AG, Montani JP (2008) Sugary drinks in the pathogenesis of obesity and cardiovascular diseases. Int J Obes 32:S28–S34
Crescenzo R, Bianco F, Falcone I, Prisco M, Liverini G, Iossa S (2008) Alterations in hepatic mitochondrial compartment in a model of obesity and insulin resistance. Obesity 16(5):958–964
Serviddio G, Bellanti F, Vendemiale G, Altomare E (2011) Mitochondrial dysfunction in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol 5(2):233–244
Patti ME, Corvera S (2010) The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev 31(3):364–395
Johannsen DL, Ravussin E (2009) The role of mitochondria in health and disease. Curr Opin Pharmacol 9(6):780–786
Elia M, Livesey G (1988) Theory and validity of indirect calorimetry during net lipid synthesis. Am J Clin Nutr 47:591–607
Kovacs EMR, Westerterp-Plantenga MS (2006) Effect of (-)-hydroxycitrate on net fat synthesis as de novo lipogenesis. Physiol Behav 88:371–381
Even PC, Mokhtarian A, Pele A (1994) Practical aspects of indirect calorimetry in laboratory animals. Neurosci Biobehav Rev 18:435–447
Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP (2008) Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol 295:E1269–E1276
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–510
Dulloo AG, Girardier L (1992) Influence of dietary composition on energy expenditure during recovery of body weight in the rat: implications for catch-up growth and obesity relapse. Metab 41:1336–1342
Armsby HP (1917) The nutrition of farm animals. The Macmillan Company, New York
Roehrig KL, Allred JB (1974) Direct enzymatic procedure for the determination of liver glycogen. Anal Biochem 58:414–421
Iossa S, Lionetti L, Mollica MP, Crescenzo R, Botta M, Barletta A, Liverini G (2003) Effect of high-fat feeding on metabolic efficiency and mitochondrial oxidative capacity in adult rats. Br J Nutr 90:953–960
Strittmatter P, Spatz L, Corcoran D, Rogers MJ, Setlow B, Redline R (1974) Purification and properties of rat liver microsomal stearyl coenzyme A desaturase. Proc Natl Acad Sci 71:4565–4569
Penicaud L, Ferre P, Assimacopoulos-Jeannet F, Perdereau D, Leturque A, Jeanrenaud B, Picon L, Girard J (1991) Increased gene expression of lipogenic enzymes and glucose transporter in white adipose tissue of suckling and weaned obese Zucker rats. Biochem J 279:303–308
Cairns CB, Walther J, Harken AH, Banerjee A (1998) Mitochondrial oxidative phosphorylation efficiencies reflect physiological organ roles. Am J Physiol 274:R1376–R1383
Crescenzo R, Bianco F, Falcone I, Prisco M, Dulloo AG, Liverini G, Iossa S (2010) Hepatic mitochondrial energetics during catch-up fat after caloric restriction. Metab 59:1221–1230
Barré H, Bailly L, Rouanet JL (1997) Increased oxidative capacity in skeletal muscle from cold-acclimated ducklings: a comparison with rats. Comput Biochem Physiol 88B:5119–5522
Fernandes MA, Custódio JB, Santos MS, Moreno AJ, Vicente JA (2006) Tetrandrine concentrations not affecting oxidative phosphorylation protect rat liver mitochondria from oxidative stress. Mitochondrion 6:176–185
Gardner PR (2002) Aconitase: sensitive target and measure of superoxide. Meth Enzymol 349:9–16
Flohè L, Otting F (1974) Superoxide dismutase assay. Meth Enzymol 105:93–104
Lionetti L, Mollica MP, Crescenzo R, D’Andrea E, Ferraro M, Bianco F, Liverini G, Iossa S (2007) Skeletal muscle subsarcolemmal mitochondrial dysfunction in high-fat fed rats exhibiting impaired glucose homeostasis. Int J Obes 31:1596–1604
Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ (2009) Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119(5):1322–1334
Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Havel PJ, Keim NL (2011) Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr. doi:10.1038/ejcn.2011.159
Koo HY, Wallig MA, Chung BH, Nara TY, Simon Cho BH, Nakamura MT (2008) Dietary fructose induces a wide range of genes with distinct shift in carbohydrate and lipid metabolism in fed and fasted rat liver. Biochim Biophys Acta 1782:341–348
Tappy L, Randin JP, Felber JP, Chiolero R, Simonson DC, Jequier E, DeFronzo RA (1986) Comparison of thermogenic effect of fructose and glucose in normal humans. Am J Physiol 250(6 Pt 1):E718–E724
Pagliassotti MJ, Prach PA, Koppenhafer TA et al (1996) Changes in insulin action, triglycerides, and lipid composition during sucrose feeding in rats. Am J Physiol Regul Integr Comp Physiol 271:R1319–R1326
Sobrecases H, Lê KA, Bortolotti M, Schneiter P, Ith M, Kreis R, Boesch C, Tappy L (2010) Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab 36(3):244–246
Ngo Sock ET, Lê KA, Ith M, Kreis R, Boesch C, Tappy L (2010) Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br J Nutr 103(7):939–943 (Epub 2009 Nov 24)
Hellerstein MK, Schwarz JM, Neese RA (1996) Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr 16:523–557
Brown MS, Goldsein JL (2008) Selective versus total insulin resistance. Cell Metab 7:95–96
Choi SH, Ginsberg HN (2011) Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab 22:353–363
Nagai Y, Nishio Y, Nakamura T, Maegawa H, Kikkawa R, Kashiwagi A (2002) Amelioration of high fructose-induced metabolic derangements by activation of PPARα. Am J Physiol 282:E1180–E1190
Park OJ, Cesar D, Faix D, Wu K, Shackleton CH, Hellerstein MK (1992) Mechanisms of fructose-induced hypertriglyceridaemia in the rat. Activation of hepatic pyruvate dehydrogenase through inhibition of pyruvate dehydrogenase kinase. Biochem J 282:753–757
Harper ME, Green K, Brand MD (2008) The efficiency of cellular energy transduction and its implications for obesity. Ann Rev Nutr 28:13–33
Rains JL, Jain SK (2011) Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 50(5):567–575
Kannappan S, Palanisamy N, Anuradha CV (2010) Suppression of hepatic oxidative events and regulation of eNOS expression in the liver by naringenin in fructose-administered rats. Eur J Pharmacol 645(1–3):177–184
Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH (2010) The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol 7(5):251–264
Yki-Järvinen H (2010) Nutritional modulation of nonalcoholic fatty liver disease and insulin resistance: human data. Curr Opin Clin Nutr Metab Care 13(6):709–714
Acknowledgments
This work was supported by a grant from University “Federico II” of Naples. The authors thank Dr. Emilia De Santis for skilful management of animal house.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crescenzo, R., Bianco, F., Falcone, I. et al. Increased hepatic de novo lipogenesis and mitochondrial efficiency in a model of obesity induced by diets rich in fructose. Eur J Nutr 52, 537–545 (2013). https://doi.org/10.1007/s00394-012-0356-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0356-y