Abstract
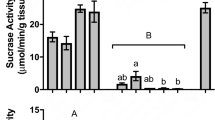
In nature, birds are faced with variable food sources that may differ in composition (protein vs. carbohydrates) and quality (highly digestible material vs. indigestible fiber). Studies in passerine birds and some commercial poultry demonstrate that the gastrointestinal tract can respond to varying diet composition and quality by changing morphology and/or activities of digestive enzymes. However, studies in additional avian species are warranted to understand generalities of these trends. We first fed juvenile mallards (Anas platyrhynchos), chickens (Gallus gallus), and quails (Coturnix coturnix) on either high-carbohydrate or high-protein diets. For the most part, birds fed the high-carbohydrate diet had higher small intestinal and cecal disaccharidase activities (maltase and sucrase). However, only mallards exhibited higher small intestinal aminopeptidase-N (APN) activities when fed the high-protein diet. These results differ from passerine birds, which largely modulate small intestinal proteases, but not disaccharidases. In another trial, we fed Canada geese (Branta canadensis) diets that varied in both their protein and fiber concentrations for approximately 3.5 months. Birds fed the high-fiber diets had significantly longer small intestines and caeca compared to those fed low-fiber diets. Additionally, geese fed the high-fiber diets exhibited lower mass-specific activities of small intestinal sucrase, and higher activities of APN when summed across the small intestine and ceca. Similar to the avian species above, geese fed the high-protein diets did not exhibit flexibility in their small intestinal APN activities. Overall, these experiments demonstrate that responsiveness of the avian digestive tract to diet composition may have phylogenetic or ecological constraints. Studies on other avian taxa are needed to understand these patterns.




Similar content being viewed by others
References
Afik D, Caviedes-Vidal E, Martínez del Rio C, Karasov WH (1995) Dietary modulation of intestinal hydrolytic enzymes in yellow-rumped warblers. Am J Physiol 269:R413–R420
Baeza E, Arnould C, Jlali M, Chartrin P, Gigaud V, Mercerand F, Durand C, Meteau K, Le Bihan-Duval E, Berri C (2012) Influence of increasing slaughter age of chickens on meat quality, welfare, and technical and economic results. J Anim Sci 90:2003–2013
Biviano AB, Martínez del Rio C, Phillips DL (1993) Ontogenesis of intestine morphology and intestinal disaccharidases in chickens (Gallus gallus) fed contrasting purified diets. J Comp Physiol B 163:508–518
Brzęk P, Caviedes-Vidal E, Karasov WH (2010a) House sparrow fledglings leave the nest digestively immature but more flexible than adults. Integr Comp Biol 50:E19–E19
Brzęk P, Lessner KM, Caviedes-Vidal E, Karasov WH (2010b) Low plasticity in digestive physiology constrains feeding ecology in diet specialist, zebra finch (Taeniopygia guttata). J Exp Biol 213:798–807
Camci O, Erensayin C, Aktan S (2002) Relations between age at sexual maturity and some production characteristics in quails. Archiv für Geflügelkunde 66:280–282
Caviedes-Vidal E, Afik D, Martínez del Rio C, Karasov WH (2000) Dietary modulation of intestinal enzymes of the house sparrow (Passer domesticus): testing an adaptive hypothesis. Comp Biom Physiol A 125:11–24
Chinery R, Goodlad RA, Wright NA (1992) Soy polysaccharide in an enteral diet: effects on rat intestinal cell proliferation, morphology and metabolic function. Clin Nutr 11:277–283
Ciminari ME, del Valle Moyano G, Chediack JG, Caviedes-Vidal E (2005) Feral pigeons in urban environments: dietary flexibility and enzymatic digestion? Rev Chil Hist Nat 78:267–279
Ciminari ME, Chediack JG, Caviedes-Vidal E (2014) Effect of dietary protein and carbohydrate content on growth and enzymatic digestion in chickens. Asian J Poult Sci 8:49–63
Clench MH, Mathias JR (1995) The avian cecum: a review. Wilson Bull 107:93–121
Dahlqvist A (1984) Assay of intestinal disaccharidases. Scand J Clin Lab Invest 44:169–172
Diamond JM (1993) Evolutionary physiology. In: Boyd CAR, Noble D (eds) Logic of Life: The Challenge of Integrative Physiology. Oxford Press, New York, pp 89–111
Diamond JM, Hammond K (1992) The matches, achieved by natural selection, between biological capacities and their natural loads. Experientia 48:551–557
Dobush GR, Ankney CD, Krementz DG (1985) The effect of apparatus, extraction time, and solvent type on lipid extractions of snow geese. Can J Zool 63:1917–1920
Foye OT, Black BL (2006) Intestinal adaptation to diet in the young domestic and wild turkey (Meleagris gallopavo). Comp Biochem Physiol A 143:184–192
Goering HK, Van Soest PJ (1970) Forage fiber analysis, vol 379. US Government Printing Office, Washington, DC
Hedemann MS, Eskildsen M, Lærke HN, Pedersen C, Lindberg JE, Laurinen P, Bach Knudsen KE (2006) Intestinal morphology and enzymatic activity in newly weaned pigs fed contrasting fiber concentrations and fiber properties. J Anim Sci 84:1375–1386
Iji PA, Saki AA, Tivey DR (2001) Intestinal structure and function of broiler chickens on diets supplemented with a mannan oligosaccharide. J Sci Food Agric 81:1186–1192
Karasov WH (1990) Digestion in birds: chemical and physiological determinants and ecological implications. Stud Avian Biol 13:391–415
Karasov WH, Martinez del Rio C (2007) Physiological ecology: how animals process energy, nutrients, and toxins. Princeton University Press, Princeton
Kehoe FP, Ankney CD, Alisauskas RT (1988) Effects of dietary fiber and diet diversity on digestive organs of captive Mallards (Anas platyrhynchos). Can J Zool 66:1597–1602
Khokhar S (1994) Dietary fibers: their effects on intestinal digestive enzyme activities. J Nutr Biochem 5:176–180
Lang CA (1958) Simple microdetermination of Kjeldahl nitrogen in biological materials. Anal Chem 30:1692–1694
Lepkovsky S, Wagner M, Furuta F, Ozone K, Koike T (1964) The proteases, amylase and lipase of the intestinal contents of germfree and conventional chickens. Poult Sci 43:722–726
Levey DJ, Place AR, Rey PJ, Martínez del Rio C (1999) An experimental test of dietary enzyme modulation in pine warblers Dendroica pinus. Physiol Biochem Zool 72:576–587
Maldonado K, Bozinovic F, Rojas JM, Sabat P (2011) Within-species digestive tract flexibility in rufous-collared sparrows and the climatic variability hypothesis. Physiol Biochem Zool 84:377–384
Maroux S, Louvard D, Barath J (1973) The aminopeptidase from hog intestinal brush border. Biochim Biophys Acta Enzymol 321:282–295
Martínez del Rio C (1990) Dietary, phylogenetic, and ecological correlates of intestinal sucrase and maltase activity in birds. Physiol Zool 63:987–1011
Martínez del Rio C, Brugger KE, Rios JL, Vergara ME, Witmer M (1995) An experimental and comparative study of dietary modulation of intestinal enzymes in European starlings (Sturnus vulgaris). Physiol Zool 68:490–511
McLandress MR, Raveling DG (1981) Changes in diet and body composition of Canada geese before spring migration. Auk 98:65–79
McLelland J (1989) Anatomy of the avian cecum. J Exp Biol 252(S3):2–9
McWhorter TJ, Caviedes-Vidal E, Karasov WH (2009) The integration of digestion and osmoregulation in the avian gut. Biol Rev 84:533–565
McWilliams SR (1999) Digestive strategies of avian herbivores. In: Adams NJ, Slotow RH (eds) XXII international ornithological congress, Durban, pp 2198–2207
Montagne L, Pluske JR, Hampson DJ (2003) A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Tech 108:95–117
Murphy ME, King JR (1982) Semi-synthetic diets as a tool for nutritional ecology. Auk 99:165–167
Obst BS, Diamond JM (1989) Interspecific variation in sugar and amino acid transport by the avian cecum. J Exp Zool 252(S3):117–126
Parrish J (2000) Behavioral, energetic, and conservation implications for foraging plasticity during migration. Stud Avian Biol 20:53–70
Richman SE, Leafloor JO, Karasov WH, McWilliams SR (2015) Ecological implications of reduced forage quality on growth and survival of sympatric geese. J Anim Ecol 84:284–298
Roberts MJ, Russo R (1999) A student’s guide to analysis of variance. Routledge, New York
Sabat P, Novoa F, Bozinovic F, Martínez del Rio C (1998) Dietary flexibility and intestinal plasticity in birds: a field and laboratory study. Physiol Biochem Zool 71:226–236
Savory CJ, Gentle MJ (1976) Changes in food intake and gut size in Japanese quail in response to manipulation of dietary fibre content. Brit Poult Sci 17:571–580
Sedinger J (1997) Adaptations to and consequences of an herbivorous diet in grouse and waterfowl. Condor 99:314–326
Sell JL, Koldovsky O, Reid BL (1989) Intestinal disaccharidases of young turkeys: temporal development and influence of diet composition. Poult Sci 68:265–277
Siddons RC (1972) Effect of diet on disaccharidase activity in the chick. Brit J Nutr 27:343–352
Thomsen LL, Tasman-Jones C (1982) Disaccharidase levels of the rat jejunum are altered by dietary fibre. Digestion 23:253–258
Undersander D, Mertens DR, Thiex N (1993) Forage analysis procedures. Omaha, NE
Williamson SE, Jones SKC, Munn AJ (2014) Is gastrointestinal plasticity in king quail (Coturnix chinensis) elicited by diet-fibre or diet-energy dilution? J Exp Biol 217:1839–1842
Yang Y, Iji PA, Kocher A, Mikkelsen LL, Choct M (2007) Effects of mannanoligosaccharide on growth performance, the development of gut microflora, and gut function of broiler chickens raised on new litter. J Appl Poult Res 16:280–288
Acknowledgments
We thank the following individuals for their help in collecting eggs in Akimiski Island: Ken Abraham, Dan Byers, Mike Donovan, Bert French, Mike Hill, Leslie Jaeger, Cynthia Kapke, Art Smith, Chris Swannell, Scott Taylor, Gary Tupling, and Roel Teunisson. We wish to thank Ciaran Hannan, Chris Jungbluth, Ann Normington (Voek), and Bettina Potts for their assistance with care and measurements of birds. Funding was in part provided by the Ontario Ministry of Natural Resources and state and federal agencies of the Mississippi and Atlantic flyways. Funding was provided by the National Science Foundation (IBN-9318675 to W.H.K. and DBI 1400456 to K.D.K.) and the Universidad Nacional de San Luis—Ciencia y Técnica Grant 2-0814 and CONICET PIP 2015-834 to E.C.-V.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kohl, K.D., Ciminari, M.E., Chediack, J.G. et al. Modulation of digestive enzyme activities in the avian digestive tract in relation to diet composition and quality. J Comp Physiol B 187, 339–351 (2017). https://doi.org/10.1007/s00360-016-1037-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-1037-6