Abstract
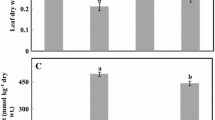
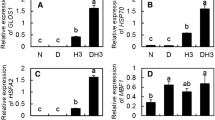
In this study we evaluated the contrasting major physiological responses of Jatropha curcas L. to salinity alone and in combination with high temperature. The plants were subjected to salinity (100 mM NaCl) before and after exposure to 43 °C (heat stress) for 6 h. The effects of salinity were more harmful than heat stress, and the effects of salt stress were increased when both stress factors were combined. The negative effects of the combined treatments included strong impairment of the CO2 assimilation rate and stomata conductance and increased Na+ and Cl− accumulation in the leaves associated with increased membrane damage and lipid peroxidation. Heat favorably stimulated the accumulation of glycine betaine and chlorophyll in the salt-stressed leaves. Treatments with salt, heat, and their combination stimulated the antioxidant enzymatic defense system, that is, the expression of ascorbate peroxidase (APX) and superoxide dismutase (SOD), whereas the expression of catalase (CAT) was stimulated through treatments with salt alone and in combination with heat; treatment with heat alone did not affect CAT expression. The ascorbate redox state was decreased under salinity stress alone and in combination with heat but remained unaffected when treated with heat alone. Overall, the leaf H2O2 concentration did not change in response to these stresses, but lipid peroxidation and membrane damage was increased. Moreover, high temperature increases the negative effects of salt stress on key physiological processes, but treatment with heat alone is favorable for several metabolic indicators of young J. curcas plants. In contrast with heat, these plants exhibit higher physiological disturbances under isolated salinity stress.



Similar content being viewed by others
References
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Ashraf M, Ali Q (2008) Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Environ Exp Bot 63:266–273
Cavalcanti FR, Oliveira JTA, Martins-Miranda AS, Viégas RA, Silveira JAG (2004) Superoxide dismutase, catalase and peroxide activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol 163:563–571
Cavalcanti FR, Lima JPMS, Ferreira-Silva SL, Viégas RA, Silveira JAG (2007) Roots and leaves display contrasting oxidative response during salt stress and recovery in cowpea. J Plant Physiol 164:591–600
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 125:1–10
Cheeseman JM (2006) Hydrogen peroxide concentrations in leaves under natural conditions. J Exp Bot 57:2435–2444
Couée I, Sulmon C, Gouesbet G, Amrani AEl (2006) Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot 57:449–459
Crafts-Brandner SJ, Salvucci ME (2002) Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol 129:1773–1780
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Dutra ATB, Silva EN, Rodrigues CRF, Vieira SA, Aragão RM, Silveira JAG (2011) High temperatures affect ion distribution in NaCl-pretreated cowpea plants. Rev Brás Eng Agr Amb 15:403–409
Ferreira-Silva SL, Voigt EL, Silva EN, Maia JM, Fontenele AV, Silveira JAG (2011) High temperature positively modulates oxidative protection in salt-stressed cashew plants. Environ Exp Bot 74:162–170
Flexas J, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbo M (2007) Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ 30:1284–1298
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55:307–319
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Giannopolotis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Gil SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Girija C, Smith BN, Swamy PM (2002) Interactive effects of sodium chloride and calcium chloride on the accumulation of proline and glycine betaine in peanut (Arachis hypogaea L.). Environ Exp Bot 47:1–10
Guerfel M, Ouni Y, Boujnah D, Zarrouk M (2009) Photosynthesis parameters and activities of enzymes of oxidative stress in two young ‘Chemlali’ and ‘Chetoui’ olive trees under water deficit. Photosynthetica 47:340–346
Guo YP, Zhou HF, Zhang LG (2006) Photosynthetic characteristics and protective mechanisms against photooxidation during high temperature stress in two citrus species. Sci Hortic 108:260–267
Havir EA, Mchale NA (1987) Biochemical and development characterization of multiples forms of catalase in tobacco leaves. Plant Physiol 84:450–455
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hessine K, Martínez JP, Gandour M, Albouchi A, Soltani A, Abdelly C (2009) Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environ Exp Bot 67:312–319
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:1–39
Kampfenkel K, Montagu MV, Inzé R (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225:165–167
Kant P, Gordon M, Kant S, Zolla G, Davydov O, Heimer YM, Chalifa-Caspi V, Shaked R, Barak S (2008) Functional-genomics-based identification of genes that regulate Arabidopsis responses to multiple abiotic stresses. Plant Cell Environ 31:697–714
Kheira AAA, Atta NMM (2009) Response of Jatropha curcas L. to water deficits: yield, water use efficiency and oilseed characteristics. Biomass Bioenergy 33:1343–1350
Kotak S, Larkindale J, Lee U, Koskull-Doring PV, Vierling E, Scharf KD (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10:310–316
Larher FR, Lugan R, Gagneul D, Guyot S, Monnier C, Lespinasse Y, Bouchereau A (2009) A reassessment of the prevalent organic solutes constitutively accumulated and potentially involved in osmotic adjustment in pear leaves. Environ Exp Bot 66:230–241
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Ma YH, Ma FW, Zhang JK, Li MJ, Wang YH, Liang D (2008) Effects of high temperature on activities and gene expression of enzymes involved in ascorbate–glutathione cycle in apple leaves. Plant Sci 175:761–766
McCormick AJ, Watt DA, Cramer MD (2009) Supply and demand: sink regulation of sugar accumulation in sugarcane. J Exp Bot 60:357–364
Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133:481–489
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 33:453–467
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Environ 22:1068–1072
Ribeiro RV, Machado EC, Oliveira RF (2004) Growth- and leaf-temperature effects on photosynthesis of sweet orange seedlings infected with Xylella fastidiosa. Plant Pathol 53:334–340
Ribeiro RV, Machado EC, Santos MG, Oliveira RF (2009a) Seasonal and diurnal changes in photosynthetic limitation of young sweet orange trees. Environ Exp Bot 66:203–211
Ribeiro RV, Machado EC, Santos MG, Oliveira RF (2009b) Photosynthesis and water relations of well-watered orange plants as affected by winter and summer conditions. Photosynthetica 47:215–222
Salvucci ME, Crafts-Brandner SJ (2004) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120:179–186
Shabala S, Cuin TA (2007) Potassium transport and plant salt tolerance. Physiol Plant 133:651–669
Silva EN, Silveira JAG, Rodrigues CRF, Dutra ATB, Aragão RM (2009a) Ion uptake and growth of physic nut under different salinity levels. Rev Ciênc Agron 40:240–246
Silva EN, Silveira JAG, Rodrigues CRF, Lima CS, Viégas RA (2009b) Contribution of organic and inorganic solutes to osmotic adjustment of physic nut under salinity. Pesqui Agropecu Bras 44:437–445
Silva EN, Ferreira-Silva SL, Fontenele AV, Ribeiro RV, Viégas RA, Silveira JAG (2010a) Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J Plant Physiol 167:1157–1164
Silva EN, Ferreira-Silva SL, Viégas RA, Silveira JAG (2010b) The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environ Exp Bot 69:279–285
Silveira JAG, Viegas RA, Rocha IMA, Moreira ACDM, Moreira RA, Oliveira JTA (2003) Proline accumulation and glutamine synthetase activity are increased by salt-induced proteolysis in cashew leaves. J Plant Physiol 160:115–123
Silveira JAG, Araújo SAM, Lima JPMS, Viégas RA (2009) Roots and leaves display contrasting osmotic adjustment mechanisms in response to NaCl-salinity in Atriplex numularia. Environ Exp Bot 66:1–8
Suzuki N, Rizhsky L, Liang H, Shuman J, Shulaev V, Mittler R (2005) Enhanced tolerance to environmental stress in transgenic plants expressing the transcriptional co-activator Multiprotein Bridging Factor 1c. Plant Physiol 139:1313–1322
Tian J, Belanger FC, Huang B (2009) Identification of heat stress-responsive genes in heat-adapted thermal Agrostis scabra by suppression subtractive hybridization. J Plant Physiol 166:588–601
Wahid A, Close TJ (2007) Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol Plant 51:104–109
Zimmermam P, Heinlein C, Orendi G, Zentgra U (2006) Senescence-specific regulation of catalases in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ 29:1049–1060
Acknowledgments
We thank the Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico - FUNCAP (Project 2155/PRONEX) and the Conselho Nacional de Desenvolvimento Científico e Tecnolígico (CNPq) for financial support. The authors would like to thank the Fazenda Tamanduá for supplying of the Jatropha curcas seeds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, E.N., Vieira, S.A., Ribeiro, R.V. et al. Contrasting Physiological Responses of Jatropha curcas Plants to Single and Combined Stresses of Salinity and Heat. J Plant Growth Regul 32, 159–169 (2013). https://doi.org/10.1007/s00344-012-9287-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-012-9287-3