Abstract
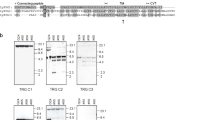
The genomic and cDNA sequences of the CD3γ/δ and CD3ɛ homologues in the mandarin fish, Siniperca chuats i, were determined. As in other vertebrate CD3 molecules, the deduced amino acid sequences of mandarin fish CD3γ/δ and CD3ɛ contained conserved residues and motifs, such as cysteine residues and CXXC and immunoreceptor tyrosine-based activation motifs. However, mandarin fish CD3γ/δ and CD3ɛ showed some differences to their mammalian counterparts, specifically the absence of a negatively charged residue in the transmembrane region of CD3γ/δ. Additionally, while an N -glycosylation site was present in CD3ɛ, the site was not observed in CD3γ/δ. The CD3γ/δ and CD3ɛ subunit sequences contain six and five exons, respectively, consistent with homologues from Atlantic salmon, Salmo salar. Phylogenetic analysis also revealed that CD3γ/δ and CD3ɛ in mandarin fish are closely related to their counterparts in Acanthopterygian fish. Real-time PCR showed CD3γ/δ and CD3ɛ were expressed mainly in the thymus and spleen in normal healthy fish and, to a lesser extent, in mucosal-associated lymphoid tissues, such as the intestine and gills. When lymphocytes isolated from head kidney were treated with the mitogens phytohemagglutinin, concanavalin, and polyriboinosinic polyribocytidylic acid, mRNA expression levels of CD3γ/δ and CD3ɛ were significantly elevated within 12 h of treatment. This indicated the presence of T lymphocytes in the head kidney of teleost fish, and also the recognition of mitogens by the lymphocytes. Mandarin fish infected with the bacterial pathogen Flavobacterium columnare also showed an increase in the expression of CD3γ/δ and CD3ɛ mRNA, indicating that CD3γ/δ and CD3ɛ lymphocytes are involved in the immune response of this species.
Similar content being viewed by others
References
Abelli L, Picchietti S, Romano N, Mastrolia L, Scapigliati G. 1997. Immunohistochemistry of gut-associated lymphoid tissue of the sea bass Dicentrarchus labrax (L.). Fish Shellfish Immunol., 7(4): 235–245.
Alabyev B Y, Guselnikov S V, Najakshin A M, Mechetina L V, Taranin A V. 2000. CD3ɛ homologues in the chondrostean fish Acipenser ruthenus. Immunogenetics, 51(12): 1 012–1 020.
André S, Kerfourn F, Fellah J S. 2011. Molecular and biochemical characterization of the Mexican axolotl CD3 (CD3ɛ and CD3γ/δ). Immunogenetics, 63(12): 847–853.
Araki k, Suetake H, Kikuchi K, Suzuki Y. 2005. Characterization and expression analysis of CD3ɛ and CD3γ/δ in fugu, Takifugu rubripes. Immunogenetics, 57(1–2): 158–163.
Bernard D, Six A, Rigottier-Gois L, Messiaen S, Chilmonczyk S, Quillet E, Boudinot P, Benmansour A. 2006. Phenotypic and functional similarity of gut intraepithelial and systemic T cells in a teleost fish. J. Immunol., 176(7): 3 942–3 949.
Bernot A, Auffray C. 1991. Primary structure and ontogeny of an avian CD3 transcript. Proc. Natl. Acad. Sci. USA., 88(6): 2 550–2 554.
Blom N, Gammeltoft S, Brunak S. 1999. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol., 294(5): 1 351–1 362.
Buonocore F, Randelli E, Casani D, Guerra L, Picchietti S, Costantini S, Facchiano A M, Zou J, Secombes C J, Scapigliati G. 2008. A CD4 homologue in sea bass (Dicentrarchus labrax): molecular characterization and structural analysis. Mol. Immunol., 45(11): 3 168–3 177.
Call M E, Wucherpfennig K W. 2005. The T cell receptor: critical role of the membrane environment in receptor assembly and function. Annu. Rev. Immunol., 23: 101–125.
Call M E, Wucherpfennig K W. 2007. Common themes in the assembly and architecture of activating immune receptors. Nat. Rev. Immunol., 7(11): 841–850.
Cambier J C. 1995. Antigen and Fc receptor signaling: the awesome power of the immunoreceptor tyrosine-based activation motif (ITAM). J. Immunol., 155(7): 3 281–3 285.
Castro R, Bernard D, Lefranc M P, Six A, Benmansour A, Boudinot P. 2011. T cell diversity and TcR repertoires in teleost fish. Fish Shellfish Immunol., 31(5): 644–654.
Chen D L, Guo X G, Nie P. 2010. Phylogenetic studies of sinipercid fish (Perciformes: Sinipercidae) based on multiple genes, with first application of an immunerelated gene, the virus-induced protein (viperin) gene. Mol. Phylogenet. Evol., 55(3): 1 167–1 176.
Chen H, Kshirsagar S, Jensen I, Lau K, Covarrubias R, Schluter S F, Marchalonis J J. 2009. Characterization of arrangement and expression of the T cell receptor gamma locus in the sandbar shark. Proc. Natl. Acad. Sci. USA., 106(21): 8 591–8 596.
Dalmo R A, Ingebrigtsen K, J Bøgwald J. 1997. Non-specific defence mechanisms in fish, with particular reference to the reticuloendothelial system (RES). J. Fish Dis. 20(4): 241–273.
Dave V P. 2009. Hierarchical role of CD3 chains in thymocyte development. Immunol. Rev., 232(1): 22–33.
Davis M M, Chien Y H. 2003. T-cell antigen receptors. In: Paul W E ed. Fundamental Immunology. 5th Edition. Lippincott Williams & Wilkins Publishers, Philadelphia, USA. p.27–258.
Decostere A, Haesebrouck F, Devriese L A. 1997. Shieh medium supplemented with tobramycin for selective isolation of Flavobacterium columnare (Flexibacter columnaris) from diseased fish. J. Clin. Microbiol., 35(1): 322–324.
Dietrich J, Neisig A, Hou X, Wegener A W, Gajhede M, Geisler C. 1996. Role of CD3 gamma in T cell receptor assembly. J. Cell Biol., 132(3): 299–310.
Dzialo R C, Cooper M D. 1997. An amphibian homologue of the mammalian CD3 γ and δ genes. Eur. J. Immunol., 27(7): 1 640–1 647.
Göbel T W, Dangy J P. 2000. Evidence for a stepwise evolution of the CD3 family. J. Immunol., 164(2): 879–883.
Gold D P, Clevers H, Alarcon B, Dunlap S, Novotnyt J, Williams A F, Terhorst C. 1987. Evolutionary relationship between the T3 chains of the T-cell receptor complex and the immunoglobulin supergene family. Proc. Natl. Acad. Sci. USA, 84(21): 7 649–7 653.
Guo Z, Nie P. 2011. The cDNA sequence and expression analysis of CD4 in mandarin fish (Siniperca chuatsi). Journal of Fisheries of China, 35(8): 1 121–1 129. (in Chinese with English abstract)
Guo Z, Wang G L, Fu J P, Nie P. Characterization and expression of CD8 molecules in mandarin fish Siniperca chuatsi. J. Fish Biol. accepted.
Jósefsson S, Tatner M F. 1993. Histogenesis of the lymphoid organs in sea bream (Sparus aurata L.). Fish Shellfish Immunol., 3(1): 35–49.
Laing K J, Zou J J, Purcell M K, Phillips R, Secombes C J, Hansen J. 2006. Evolution of the CD4 family: teleost fish possess two divergent forms of CD4 in addition to lymphocyte activation gene-3. J. Immunol., 177(6): 3 939–3 951.
Laing K J, Hansen J D. 2011. Fish T cells: recent advances through genomics. Dev. Com. Immunol., 35(11): 1 282–1 295.
Letunic I, Doerks T, Bork P. 2012. SMART 7: recent updates to the protein domain annotation resourse. Nucleic Acids Res., 40: 302–305.
Liu Y, Moore L, Koppang E O, Hordvik I. 2008. Characterization of the CD3ζ, CD3γ/δ and CD3ɛ subunits of the T cell receptor complex in Atlantic salmon. Dev. Comp. Immunol., 332 (1): 26–35.
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25(4): 402–408.
Mäkelä S M, Osterlund P, Julkunen I. 2011. TLR ligands induce synergistic interferon-β and interferon-λ1 gene expression in human monocyte-derived dendritic cells. Mol. Immunol., 48(4): 505–515.
Maisey K, Imarai M. 2011. Diversity of teleost leukocyte molecules: Role of alternative splicing. Fish Shellfish Immunol., 31(5): 663–672.
Matsunaga T, Rahman A. 2001. In search of the origin of the thymus: the thymus and GALT may be evolutionarily related. Scand. J. Immunol., 53(1): 1–6.
McClelland E K, Ming T J, Tabata A, Miller K M. 2011. Sequence analysis of MHC class I α2 from sockeye salmon (Oncorhynchus nerka). Fish Shellfish Immunol., 31(3): 507–510.
McMillan N D, Secombes C. 1997. Isolation of rainbow trout (Oncorhynchus mykiss) intestinal intraepithelial lymphocytes (IEL) and measurement of their cytotoxic activity. Fish Shellfish Immunol., 7(8): 527–541.
Meeker N D, Smith A C, Frazer J K, Bradley D F, Rudner L A, Love C, Trede N S. 2010. Characterization of the zebrafish T cell receptor beta locus. Immunogenetics, 62(1): 23–29.
Miller J B, Hsu R, Heinrikson R, Yachnin S. 1975. Extensive homology between the subunits of the phytohemagglutinin mitogenic proteins derived from Phaseolus vulgaris. Proc. Natl. Acad. Sci. USA., 72(4): 1 388–1 391.
Miyara M, Sakaguchi S. 2007. Natural regulatory T cells: mechanisms of suppression. Trends Mol. Med., 13(3): 108–116.
Minami Y, Weissman A M, Samelson L E, Klausner R D. 1987. Building a multichain receptor: synthesis, degradation, and assembly of the T-cell antigen receptor. Proc. Natl. Acad. Sci. USA., 84(9): 2 688–2 692.
Moore L J, Mijkstra J M, Koppang E O, Hordvik I. 2009. CD4 homologues in Atlantic salmon. Fish Shellfish Immunol., 26(1): 10–18.
Øvergård A C, Hordvik I, Nerland A H, Eikeland G, Patel S. 2009. Cloning and expression analysis of Atlantic halibut (Hippoglossus hippoglossus) CD3 genes. Fish Shellfish Immunol., 27(6): 707–713.
øvergård A C, Nerland A H, Patel S. 2010. Cloning, characterization, and expression pattern of Atlantic halibut (Hippoglossus hippoglossus L.) CD4-2, Lck, and ZAP-70. Fish Shellfish Immunol., 29(6): 987–997.
Park C I, Hirono I, Enomoto J, Nam BH, Aoki T. 2001. Cloning of Japanese flounder Paralichthys olivaceus CD3 cDNA and gene, and analysis of its expression. Immunogenetics, 53(2): 130–135.
Park C I, Hirono I, Aoki T. 2005. Molecular characterization of the Japanese flounder Paralichthys olivaceus CD3ɛ and evolution of the CD3 cluster. Dev. Comp. Immunol., 29(2): 123–133.
Patel S, Overgård A C, Nerland A H. 2009. A CD4 homologue in Atlantic halibut (Hippoglossus hippoglossus): molecular cloning and characterisation. Fish Shellfish Immunol., 26(3): 377–384.
Petersen T N, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods, 8(10): 785–786.
Picchietti S, Guerra L, Buonocore F, Randelli E, Fausto A M, Abelli L. 2009. Lymphocyte differentiation in sea bass thymus: CD4 and CD8-α gene expression studies. Fish Shellfish Immunol., 27(1): 50–56.
Picchietti S, Guerra L, Bertoni F, Randelli E, Belardinelli M C, Buonocore F, Fausto A M, Rombout J H, Scapigliati G, Abelli L. 2011. Intestinal T cells of Dicentrarchus labrax (L.): gene expression and functional studies. Fish Shellfish Immunol., 30(2): 609–617.
Pridgeon J W, Klesius P H. 2010. Identification and expression profile of multiple genes in channel catfish fry 10 min after modified live Flavobacterium columnare vaccination. Vet. Immunol. Immunopathol., 138(1–2): 25–33.
Quiniou S M A, Sahoo M, Edholm E-S, Bengten E, Wilson M. 2011. Channel catfish CD8α and CDβ co-receptors: characteriztion, expression and polymorphism. Fish Shellfish Immunol., 30(3): 894–901.
Romano N, Rossi F, Abelli L, Caccia E, Piergentili R, Mastrolia L, Randelli E, Buonocore F. 2007. Majority of TcRβ (+) T-lymphocytes located in the thymus and midgut of the bony fish, Dicentrarchus labrax (L.). Cell Tiss. Res., 329(3): 479–489.
Rombout J H W M, Huttenhuis H B T, Picchietti S, Scapigliati G. 2005. Phylogeny and ontogeny of fish leucocytes. Fish Shellfish Immunol., 19(5): 441–455.
Rombout J H W M, Abelli L, Simona Picchietti S, Giuseppe Scapigliati G, Viswanath Kiron V. 2011. Teleost intestinal immunology. Fish Shellfish Immunol., 31(5): 616–626.
Ropars A, Bautz A M, Doumon C. 2002. Sequencing and expression of CD3γ/δ mRNA in Pleurodeles waltl (urodele amphibian). Immunogenetics, 54(2): 130–138.
Rudd P M, Elliott T, Cresswell P, Wilson I A, Dwek R A. 2001. Glycosylation and the immune system. Science, 291(5 512): 2 370–2 376.
Schneck J L, Caslake L F. 2006. Genetic diversity of Flavobacterium columnare isolated from fish collected from warm and cold water. J. Fish Dis., 29(4): 245–248.
Saito H, Koyama T, Georgopoulos K, Clevers H, Haser W G, LeBien T, Tonegawa S, Terhorst C. 1987. Close linkage of the mouse and human CD3 gamma- and delta-chain genes suggests that their transcription is controlled by common regulatory elements. Proc. Natl. Acad. Sci. USA., 84(24): 9 131–9 134.
Shang N, Sun X F, Hu W, Wang Y P, Guo Q L. 2008. Molecular cloning and characterization of common carp (Cyprinus carpio L.) TCRγ and CD3γ/δ chains. Fish Shellfish Immunol., 24(4): 412–425.
Shen P, Fan X R, Li G W. 1989. Experiment in microbiology, 3rd. Higher Education Press, Beijing, China. p.92–95. (in Chinese)
Shibasaki Y, Toda H, Kobayashi I, Moritomo T, Nakanishi T. 2010. Kinetics of CD4 and CD8α T-cell subsets in graftversus-host reaction (GVHR) in ginbuna crucian carp Carassius auratus langsdorfii. Dev. Comp. Immunol., 34(10): 1 075–1 081.
Sonnhammer E L, von Heijne G, Krogh A. 1998. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Bio., 6: 175–182.
Tailor P, Tsai S, Shameli A, Serra P, Wang J, Robbins S, Nagata M, Szymczak-Workman A L, Vignali D A, Santamaria P. 2008. The proline-rich sequence of CD3ɛ as an amplifier of low-avidity TCR signaling. J. Immunol., 181(1): 243–255.
Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol., 24(8): 1 596–1 599.
Thompson J D, Higgins D G, Gibson T J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res., 22: 4 673–4 680.
Tian J Y, Xie H X, Zhang Y A, Xu Z, Yao W J, Nie P. 2009a. Ontogeny of IgM-producing cells in the mandarin fish Siniperca chuatsi, identified by in situ hybridization. Vet. Immunol. Immunopathol., 132(2–4): 146–152.
Tian J Y, Sun B J, Luo Y P, Zhang Y A, Nie P. 2009b. Distribution of IgM, IgD and IgZ in mandarin fish, Siniperca chuatsi lymphoid tissues and their transcriptional changes after Flavobacterium columnare stimulation. Aquaculture, 288(1–2): 14–21.
Tian J Y, Qi Z T, Wu N, Chang M X, Nie P. cDNA sequences of the constant regions of T cell antigen receptors α, β and γ in mandarin fish (Siniperca chuatsi) and their transcriptional changes after Flavobacterium columnare stimulation. J. Fish Dis. In press.
Toda H, Saito Y, Koike T, Takizawa F, Araki K, Yabu T, Somamoto T, Suetake H, Suzuki Y, Ototake M, Moritomo T, Nakanishi T. 2011. Conservation of characteristics and functions of CD4 positive lymphocytes in a teleost fish. Dev. Comp. Immunol., 35(6): 650–660.
Toda H, Shibasaki Y, Koike T, Ohtani M, Takizawa F, Ototake M, Moritomo T, Nakanishi T. 2009. Alloantigen-specific killing is mediated by CD8-positive T cells in fish. Dev. Comp. Immunol., 33(4): 646–652.
Tunnacliffe A, Buluwela L, Rabbitts T H. 1987. Physical linkage of three CD3 genes on human chromosome 11. EMBO J., 6(10): 2 953–2 957.
Wang L F, Xie H X, Zhang J, Li N, Yao W J, Zhang L Q, Xiong C X, Nie P. 2010. Columnaris disease and genetic diversity of its bacterial pathogen Flavobacterium columnare in freshwater fish in China. Acta Hydrobiologica Sinica, 34(2): 367–377. (in Chinese with English abstract)
Xu T, Sun Y, Shi G, Cheng Y, Wang R. 2011. Characterization of the major histocompatibility complex class II genes in miiuy croaker. PLoS One, 6(8): e23823.
Xu S W, Wu J Y, Hu K S, Ping H L, Duan Z G, Zhang H F. 2010. Molecular cloning and expression of orange-spotted grouper (Epinephelus coioides) CD8α and CD8β genes. Fish Shellfish Immunol., 30(2): 600–608.
Zapata A, Cooper E L. 1990. The Immune System: Comparative Histophysiology. John Wiley & Sons, Chichester, UK. 356p.
Zapata A, Chibá A, Varas A. 1996. Cells and tissues of the immune system of fish. In: Iwama G, Nakanishi T eds. The Fish Immune System: Organism, Pathogen, and Environment. Academic Press, London. p.1–62
Zhang Y A, Nie P, Luo H Y, Wang Y P, Sun Y H, Zhu Z Y. 2003. Characterization of cdna encoding immunoglobulin light chain of the mandarin fish Siniperca chuatsi. Vet. Immunol. Immunopathol., 95(1–2): 81–90.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (No. U0631010), the Government of Guangdong Province, and the National Basic Research Program of China (973 Program) (No. 2009CB118703)
Rights and permissions
About this article
Cite this article
Guo, Z., Nie, P. Sequencing and expression analysis of CD3γ/δ and CD3ɛ chains in mandarin fish, Siniperca chuatsi . Chin. J. Ocean. Limnol. 31, 106–117 (2013). https://doi.org/10.1007/s00343-013-2018-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-013-2018-1