Abstract
Objective
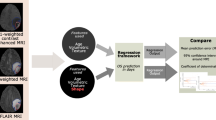
Despite 90 % of glioblastoma (GBM) recurrences occurring in the peritumoral brain zone (PBZ), its contribution in patient survival is poorly understood. The current study leverages computerized texture (i.e. radiomic) analysis to evaluate the efficacy of PBZ features from pre-operative MRI in predicting long- (>18 months) versus short-term (<7 months) survival in GBM.
Methods
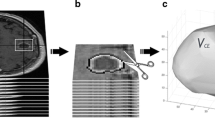
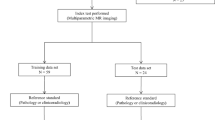
Sixty-five patient examinations (29 short-term, 36 long-term) with gadolinium-contrast T1w, FLAIR and T2w sequences from the Cancer Imaging Archive were employed. An expert manually segmented each study as: enhancing lesion, PBZ and tumour necrosis. 402 radiomic features (capturing co-occurrence, grey-level dependence and directional gradients) were obtained for each region. Evaluation was performed using threefold cross-validation, such that a subset of studies was used to select the most predictive features, and the remaining subset was used to evaluate their efficacy in predicting survival.
Results
A subset of ten radiomic ‘peritumoral’ MRI features, suggestive of intensity heterogeneity and textural patterns, was found to be predictive of survival (p = 1.47 × 10-5) as compared to features from enhancing tumour, necrotic regions and known clinical factors.
Conclusion
Our preliminary analysis suggests that radiomic features from the PBZ on routine pre-operative MRI may be predictive of long- versus short-term survival in GBM.
Key Points
• Radiomic features from peritumoral regions can capture glioblastoma heterogeneity to predict outcome.
• Peritumoral radiomics along with clinical factors are highly predictive of glioblastoma outcome.
• Identifying prognostic markers can assist in making personalized therapy decisions in glioblastoma.




Similar content being viewed by others
Change history
12 June 2017
An erratum to this article has been published.
Abbreviations
- GBM:
-
Glioblastoma multiforme
- Gd:
-
Gadolinium
- HIPAA:
-
Health Insurance Portability and Accountability Act
- KM:
-
Kaplan-Meier
- KPS:
-
Karnofsky performance score
- LTS:
-
Long-term survival
- OS:
-
Overall survival
- PBZ:
-
Peritumoral brain zone
- RF:
-
Random forest
- STS:
-
Short-term survival
- T:
-
Tesla
- TCGA:
-
The Cancer Genome Atlas
References
Krex D, Klink B, Hartmann C et al (2007) Long-term survival with glioblastoma multiforme. Brain 130:2596–2606
Osta WA, Chen Y, Mikhitarian K et al (2004) EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res 64:5818–5824
Bonavia R, Mukasa A, Narita Y et al (2010) Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev 24:1731–1745
Lemée J-M, Clavreul A, Menei P (2015) Intratumoral heterogeneity in glioblastoma: don’t forget the peritumoral brain zone. Neuro Oncol
Dehnhardt M, Zoriy MV, Khan Z et al (2008) Element distribution is altered in a zone surrounding human glioblastoma multiforme. J Trace Elem Med Biol 22:17–23
Engelhorn T, Savaskan NE, Schwarz MA et al (2009) Cellular characterization of the peritumoral edema zone in malignant brain tumors. Cancer Sci 100:1856–1862
Aubry M, de Tayrac M, Etcheverry A et al (2015) From the core to beyond the margin’: a genomic picture of glioblastoma intratumor heterogeneity. Oncotarget 6:12094
Lemée J-M, Clavreul A, Aubry M et al (2015) Characterizing the peritumoral brain zone in glioblastoma: a multidisciplinary analysis. J Neuro-Oncol 122:53–61
Badie B, Schartner JM, Hagar AR et al (2003) Microglia cyclooxygenase-2 activity in experimental gliomas possible role in cerebral edema formation. Clin Cancer Res 9:872–877
Davies D (2002) Blood–brain barrier breakdown in septic encephalopathy and brain tumours*. J Anat 200:639–646
Lin Z-X (2013) Glioma-related edema: new insight into molecular mechanisms and their clinical implications. Chin J Cancer 32:49
Itakura H, Achrol AS, Mitchell LA et al (2015) Magnetic resonance image features identify glioblastoma phenotypic subtypes with distinct molecular pathway activities. Sci Transl Med 7:303ra138
Zhang Z, Jiang H, Chen X et al (2014) Identifying the survival subtypes of glioblastoma by quantitative volumetric analysis of MRI. J Neuro-Oncol 119:207–214
Gillies RJ, Kinahan PE, Hricak H (2015) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Haralick RM, Shanmugan K, Dinstein I (1973) Textural features for image classification. IEEE Trans Syst Man Cybern SMC-3:610–621
Ryu YJ, Choi SH, Park SJ, Yun TJ, Kim J-H, Sohn C-H (2014) Glioma: application of whole-tumor texture analysis of diffusion-weighted imaging for the evaluation of tumor heterogeneity. PLoS One 9:e108335
Davnall F, Yip CS, Ljungqvist G et al (2012) Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3:573–589
O’Connor JP, Rose CJ, Waterton JC, Carano RA, Parker GJ, Jackson A (2015) Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res 21:249–257
Laws K (1980) Textured image segmentation
Clark K, Vendt B, Smith K et al (2013) The Cancer Imaging Archive (TCIA): maintaining and operating a public information repository. J Digit Imaging 26:1045–1057
Pieper S, Halle M, Kikinis R (2004) 3D SLICER. 632–635
Madabhushi A, Udupa JK (2006) New methods of MR image intensity standardization via generalized scale. Med Phys 33:3426–3434
Tao X, Chang M-C (2010) A skull stripping method using deformable surface and tissue classification. In: SPIEMedicalImaging. International Society for Optics and Photonics, p 76233L
Hammoud MA, Sawaya R, Shi W, Thall PF, Leeds NE (1996) Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neuro-Oncol 27:65–73
Prasanna P, Dana KJ, Gucunski N et al (2014) Automated crack detection on concrete bridges
Jain AK, Farrokhnia F (1991) Unsupervised texture segmentation using gabor filters. Pattern Recogn 24:1167–1186
Tiwari P, Prasanna P, Rogers L et al (2014) Texture descriptors to distinguish radiation necrosis from recurrent brain tumors on multi-parametric MRI. In: SPIEMedicalImaging. International Society for Optics and Photonics, p 90352B
De Jay N, Papillon-Cavanagh S, Olsen C, El-Hachem N, Bontempi G, Haibe-Kains B (2013) mRMRe: an R package for parallelized mRMR ensemble feature selection. Bioinformatics
Breiman L (2001) Random forests. Mach Learn 45:5–32
Tiwari P, Viswanath S, Kurhanewicz J, Sridhar A, Madabhushi A (2012) Multimodal wavelet embedding representation for data combination (MaWERiC): integrating magnetic resonance imaging and spectroscopy for prostate cancer detection. NMR Biomed 25:607–619
Shen K, Shen Z, Han Q (1997) Cox proportion hazard model multivariate analysis of prognosis of 1,484 axillary node-negative breast cancer patients. Zhonghua Zhong Liu Za Zhi 19:221–224
Mazurowski MA, Desjardins A, Malof JM (2013) Imaging descriptors improve the predictive power of survival models for glioblastoma patients. Neuro-Oncology 15:1389–1394
Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE (2014) Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol 32:774–782
Lamborn KR, Chang SM, Prados MD (2004) Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro-Oncology 6:227–235
Gandrud C (2013) Reproducible research RRstudio. CRC Press
Harrell FEJ (2001) Regression modeling Strategies applications Linearmodels, Logisticregression survival analysis. Springer Ver
Lee K, Mark D (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387
Kickingereder P, Burth S, Wick A et al (2016) Radiomic profiling of glioblastoma: identifying an imaging predictor of patient survival with improved performance over established clinical and radiologic risk models. Radiology 280:880–889
Schoenegger K, Oberndorfer S, Wuschitz B et al (2009) Peritumoral edema on MRI at initial diagnosis: an independent prognostic factor for glioblastoma? Eur J Neurol 16:874–878
Carlson MR, Pope WB, Horvath S et al (2007) Relationship between survival and edema in malignant gliomas: role of vascular endothelial growth factor and neuronal pentraxin 2. Clin Cancer Res 13:2592–2598
Carrillo J, Lai A, Nghiemphu P et al (2012) Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. Am J Neuroradiol 33:1349–1355
Lacroix M, Abi-Said D, Fourney DR et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198
Pope WB, Sayre J, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF (2005) MR imaging correlates of survival in patients with high-grade gliomas. Am J Neuroradiol 26:2466–2474
Zhou M, Hall L, Goldgof D et al (2014) Radiologically defined ecological dynamics and clinical outcomes in glioblastoma multiforme: preliminary results. Transl Oncol 7:5–13
Kraus JA, Wenghoefer M, Schmidt MC et al (2000) Long-term survival of glioblastoma multiforme: importance of histopathological reevaluation. J Neurol 247:455–460
Acknowledgments
The scientific guarantor of this publication is Dr. Anant Madabhushi (Professor, Biomedical Engineering, Case Western Reserve University: email: axm788@case.edu). The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. No complex statistical methods were necessary for this paper. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers 1U24CA199374-01, R21CA179327-01; R21CA195152-01, the National Institute of Diabetes and Digestive and Kidney Diseases under award number R01DK098503-02, the DOD Prostate Cancer Synergistic Idea Development Award (PC120857); the DOD Lung Cancer Idea Development New Investigator Award (LC130463), the DOD Prostate Cancer Idea Development Award; the Case Comprehensive Cancer Center Pilot Grant VelaSano Grant from the Cleveland Clinic, the Wallace H. Coulter Foundation Program in the Department of Biomedical Engineering at Case Western Reserve University; Ohio Third Frontier Technology Validation Award; NSF-Icorps @Ohio program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The patient cohort was obtained from The Cancer Imaging Archive (TCIA). TCIA is an open archive of cancer-specific medical images and associated clinical metadata established by the collaboration between the National Cancer Institute (NCI) and participating institutions in the United States. The HIPPA compliant project in TCGA was conducted in compliance with regulations and policies for the protection of human subjects, and approvals by institutional review boards were appropriately obtained. The cohort is used for retrospective prognostic study using multi-institutional data.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised: The captions of Figure 3 and Figure 4 were interchanged. The correct versions are given below.
An erratum to this article is available at https://doi.org/10.1007/s00330-017-4815-y.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 338 kb)
Rights and permissions
About this article
Cite this article
Prasanna, P., Patel, J., Partovi, S. et al. Radiomic features from the peritumoral brain parenchyma on treatment-naïve multi-parametric MR imaging predict long versus short-term survival in glioblastoma multiforme: Preliminary findings. Eur Radiol 27, 4188–4197 (2017). https://doi.org/10.1007/s00330-016-4637-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4637-3