Abstract
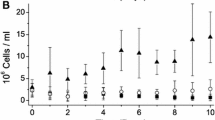
During winter in the Arctic marine ecosystem, diatoms have to survive long periods of darkness caused by low sun elevations and the presence of sea ice covered by snow. To better understand how diatoms survive in the dark, we subjected cultures of the Arctic diatom Chaetoceros neogracilis to a prolonged period of darkness (1 month) and to light resupply. Chaetoceros neogracilis was not able to grow in the dark but cell biovolume remained constant after 1 month in darkness. Rapid resumption of photosynthesis and growth recovery was also found when the cells were transferred back to light at four different light levels ranging from 5 to 154 µmol photon m−2 s−1. This demonstrates the remarkable ability of this species to re-initiate growth over a wide range of irradiances even after a prolonged period in the dark with no apparent lag period or impact on survival. Such recovery was possible because C. neogracilis cells preserved their Chl a content and their light absorption capabilities. Carbon fixation capacity was down-regulated (ninefold dark decrease in \(P_{\text{m}}^{\text{C}}\)) much more than was the photochemistry in PSII (2.3-fold dark decrease in ETRm). Rubisco content, which remained unchanged after one month in the dark, was not responsible for the decrease in \(P_{\text{m}}^{\text{C}}\). The decrease in PSII activity was partially related to the induction of sustained non-photochemical quenching (NPQ) as we observed an increase in diatoxanthin content after one month in the dark.







Similar content being viewed by others
References
Antia NJ, Cheng JY (1970) The survival of axenic cultures of marine planktonic algae from prolonged exposure to darkness at 20 °C. Phycologia 9:179–183
Arrigo KR, Mills MM, Kropuenske LR, van Dijken GL, Alderkamp A-C, Robinson DH (2010) Photophysiology in two major southern ocean phytoplankton taxa: photosynthesis and growth of Phaeocystis antarctica and Fragilariopsis cylindrus under different irradiance levels. Integr Comp Biol 50:950–966
Bailey S, Melis A, Mackey KRM, Cardol P, Finazzi G, van Dijken G, Berg GM, Arrigo K, Shrager J, Grossman A (2008) Alternative photosynthetic electron flow to oxygen in marine Synechococcus. Biochim Biophys Acta 1777:269–276
Baldisserotto C, Ferroni L, Andreoli C, Fasulo MP, Bonora A, Pancaldi S (2005) Dark-acclimation of the chloroplast in Koliella antarctica exposed to a simulated austral night condition. Arct Antarct Alp Res 37:146–156
Balzano S, Gourvil P, Siano R, Chanoine M, Marie D, Lessard S, Sarno D, Vaulot D (2012) Diversity of cultured photosynthetic flagellates in the northeast Pacific and Arctic Oceans in summer. Biogeosciences 9:4553–4571
Balzano S, Percopo I, Siano R, Gourvil P, Chanoine M, Marie D, Vaulot D, Sarno D (2017) Morphological and genetic diversity of Beaufort Sea diatoms with high contributions from the Chaetoceros neogracilis species complex. J Phycol 53:161–187
Berge J, Daase M, Renaud Paul E, AmbroseWilliam G, Darnis G, Last Kim S, Leu E, Cohen Jonathan H, Johnsen G, Moline Mark A, Cottier F, Varpe Ø, Shunatova N, Bałazy P, Morata N, Massabuau J-C, Falk-Petersen S, Kosobokova K, Hoppe Clara JM, Węsławski Jan M, Kukliński P, Legeżyńska J, Nikishina D, Cusa M, Kędra M, Włodarska-Kowalczuk M, Vogedes D, Camus L, Tran D, Michaud E, Gabrielsen Tove M, Granovitch A, Gonchar A, Krapp R, CallesenTrine A (2015) Unexpected levels of biological activity during the polar night offer new perspectives on a warming arctic. Curr Biol 25:2555–2561
Brunet C, Casotti R, Vantrepotte V, Corato F, Conversano F (2006) Picophytoplankton diversity and photoacclimation in the Strait of Sicily (Mediterranean Sea) in summer I. Mesoscale variations. Aquat Microb Ecol 44:127–141
Brunet C, Casotti R, Vantrepotte V, Conversano F (2007) Vertical variability and diel dynamics of picophytoplankton in the Strait of Sicily, Mediterranean Sea, in summer. Mar Ecol Prog Ser 346:15–26
Bunt JS, Lee CC (1972) Data on the composition and dark survival of four sea-ice microalgae. Limnol Oceanogr 17:458–461
Cardol P, Bailleul B, Rappaport F, Derelle E, Béal D, Breyton C, Bailey S, Wollman FA, Grossman A, Moreau H, Finazzi G (2008) An original adaptation of photosynthesis in the marine green alga Ostreococcus. Proc Natl Acad Sci USA 105:7881–7886
Dehning I, Tilzer MM (1989) Survival of Scenedesmus acuminatus (chlorophyceae) in darkness. J Phycol 25:509–515
Deventer B, Heckman C (1996) Effects of prolonged darkness on the relative pigment content of cultured diatoms and green algae. Aquat Sci 58:241–252
Fang X, Sommer U (2017) Overwintering effects on the spring bloom dynamics of phytoplankton. J Plankton Res 39:772–780
Ferroni L, Baldisserotto C, Zennaro V, Soldani C, Fasulo MP, Pancaldi S (2007) Acclimation to darkness in the marine chlorophyte Koliella antarctica cultured under low salinity: hypotheses on its origin in the polar environment. Eur J Phycol 42:91–104
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Goss R, Jakob T (2010) Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth Res 106:103–122
Grossman AR, Mackey KRM, Bailey S (2010) A perpective on photosynthesis in the oligotrophic oceans: hypothesis concerning alternate routes of electron flow. J Phycol 46:629–634
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of invertabrate animals. Springer, New York, pp 29–66
Hancke K, Lund-Hansen LC, Lamare ML, Højlund Pedersen S, King MD, Andersen P, Sorrell BK (2018) Extreme low light requirement for algae growth underneath sea ice: a case study from station Nord, NE Greenland. J Geophys Res 123:985–1000
Hendrickson L, Furbank R, Chow W (2004) A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth Res 82:73–81
Hughes DJ, Campbell DA, Doblin MA, Kromkamp JC, Lawrenz E, Moore CM, Oxborough K, Prášil O, Ralph PJ, Alvarez MF, Suggett DJ (2018a) Roadmaps and detours: active chlorophyll-a assessments of primary productivity across marine and freshwater systems. Environ Sci Technol 52:12039–12054
Hughes DJ, Varkey D, Doblin MA, Ingleton T, McInnes A, Ralph PJ, van Dongen-Vogels V, Suggett DJ (2018b) Impact of nitrogen availability upon the electron requirement for carbon fixation in Australian coastal phytoplankton communities. Limnol Oceanogr 63:1891–1910
Huot Y, Babin M (2010) Overview of Fluorescence protocols: theory, basic concepts, and practice. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 31–74
Jakob T, Goss R, Wilhelm C (1999) Activation of diadinoxanthin de-epoxidase due to a chiororespiratory proton gradient in the dark in the diatom Phaeodactylum tricornutum. Plant Biol 1:76–82
Kropuenske LR, Mills MM, Van Dijken GL, Bailey S, Robinson DH, Welschmeyer NA, Arrigo KR (2009) Photophysiology in two major Southern Ocean phytoplankton taxa: photoprotection in Phaeocystis antarctica and Fragilariopsis cylindrus. Limnol Ocenaogr 54:1176–1196
Kropuenske LR, Mills MM, van Dijken GL, Alderkamp A-C, Mine Berg G, Robinson DH, Welschmeyer NA, Arrigo KR (2010) Strategies and rates of photoacclimation in two major southern ocean phytoplankton taxa: Phaeocystis antarctica (Haptophyta) and Fragilariopsis cylindrus (Bacillariophyceae). J Phycol 46:1138–1151
Kvernvik AC, Hoppe CJM, Lawrenz E, Prášil O, Greenacre M, Wiktor JM, Leu E (2018) Fast reactivation of photosynthesis in arctic phytoplankton during the polar night1. J Phycol 54:461–470
Lacour T, Larivière J, Babin M (2017) Growth, Chl a content, photosynthesis, and elemental composition in polar and temperate microalgae. Limnol Oceanogr 62:43–58
Lacour T, Larivière J, Ferland J, Bruyant F, Lavaud J, Babin M (2018) The role of sustained photoprotective non-photochemical quenching in low temperature and high light acclimation in the bloom-forming arctic diatom Thalassiosira gravida. Front Mar Sci 5:354
Lavaud J, van Gorkom HJ, Etienne AL (2002) Photosystem II electron transfer cycle and chlororespiration in planktonic diatoms. Photosynth Res 74:51–59
Lavaud J, Strzepek RF, Kroth PG (2007) Photoprotection capacity differs among diatoms: possible consequences on the spatial distribution of diatoms related to fluctuations in the underwater light climate. Limnol Oceanogr 52:1188–1194
Leu E, Mundy CJ, Assmy P, Campbell K, Gabrielsen TM, Gosselin M, Juul-Pedersen T, Gradinger R (2015) Arctic spring awakening—Steering principles behind the phenology of vernal ice algal blooms. Prog Oceanogr 139:151–170
Lewis MR, Smith JC (1983) A small volume, short-incubation-time method for measure-ment of photosynthesis as a function of incident irradiance. Mar Ecol Prog Ser 13:99–102
Lüder UH, Wiencke C, Knoetzel J (2002) Acclimation of photosynthesis and pigments during and after six months of darkness in Palmaria decipiens (rhodophyta): a study to simulate antarctic winter sea ice cover. J Phycol 38:904–913
Lundholm N, Ribeiro S, Andersen TJ, Koch T, Godhe A, Ekelund F, Ellegaard M (2011) Buried alive—germination of up to a century-old marine protist resting stages. Phycologia 50:629–640
MacIntyre HL, Cullen JJ (2005) Using cultures to investigate the physiological ecology of microalgae. In: Anderson RA (ed) Algal culturing techniques. Academic Press, Sai Deigo, pp 287–326
MacIntyre HL, Sharkey TD, Geider RJ (1997) Activation and deactivation of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) in three marine microalgae. Photosynth Res 51:93–106
Mackey KRM, Paytan A, Grossman AR, Bailey S (2008) A photosynthetic strategy for coping in a high-light, low-nutrient environment. Limnol Oceanogr 53:14
Martin A, McMinn A, Heath M, Hegseth EN, Ryan KG (2012) The physiological response to increased temperature in over-wintering sea ice algae and phytoplankton in McMurdo Sound, Antarctica and Tromso Sound, Norway. J Exp Mar Biol Ecol 428:57–66
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
McMinn A, Martin A (2013) Dark survival in a warming world. Proc R Soc B 280:20122909
McMinn A, Ashworth C, Ryan K (1999) Growth and productivity of Antarctic sea ice algae under PAR and UV irradiances. Bot Mar 42:401–407
Mills MM, Kropuenske LR, van Dijken GL, Alderkamp A-C, Berg GM, Robinson DH, Welschmeyer NA, Arrigo KR (2010) Photophysiology in two southern ocean phytoplankton taxa: photosynthesis of Phaeocystis antarctica (Prymnesiophyceae) and Fragilariopsis cylindrus (Bacillariophyceae) under simulated mixed-layer irradiance. J Phycol 46:1114–1127
Morgan-Kiss RM, Priscu JC, Pocock T, Gudynaite-Savitch L, Huner NPA (2006) Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol Mol Biol Rev 70:222–252
Mundy CJ, Gosselin M, Ehn J, Gratton Y, Rossnagel A, Barber DG, Martin J, Tremblay J-É, Palmer M, Arrigo KR, Darnis G, Fortier L, Else B, Papakyriakou T (2009) Contribution of under-ice primary production to an ice-edge upwelling phytoplankton bloom in the Canadian Beaufort Sea. Geophys Res Lett 36:L17601
Murphy AM, Cowles TJ (1997) Effects of darkness on multi-excitation in vivo fluorescence and survival in a marine diatom. Limnol Oceanogr 42:1444–1453
Murphy CD, Ni G, Li G, Barnett A, Xu K, Grant-Burt J, Liefer JD, Suggett DJ, Campbell DA (2016) Quantitating active photosystem II reaction center content from fluorescence induction transients. Limnol Oceanogr 15:54–69
Murphy CD, Roodvoets MS, Austen EJ, Dolan A, Barnett A, Campbell DA (2017) Photoinactivation of photosystem II in Prochlorococcus and Synechococcus. PLoS ONE 12:e0168991
Nymark M, Valle KC, Hancke K, Winge P, Andresen K, Johnsen G, Bones AM, Brembu T (2013) Molecular and photosynthetic responses to prolonged darkness and subsequent acclimation to re-illumination in the diatom Phaeodactylum tricornutum. PLoS ONE 8:e58722
Oguchi R, Terashima I, Kou J, Chow WS (2011) Operation of dual mechanisms that both lead to photoinactivation of Photosystem II in leaves by visible light. Physiol Plant 142:47–55
Oxborough K, Moore CM, Suggett DJ, Lawson T, Chan HG, Geider RJ (2012) Direct estimation of functional PSII reaction center concentration and PSII electron flux on a volume basis: a new approach to the analysis of Fast Repetition Rate fluorometry (FRRf) data. Limnol Oceanogr 10:142–154
Palmisano AC, Sullivan CW (1982) Physiology of sea ice diatoms. I. Response of three polar diatoms to a simulated summer-winter transition. J Phycol 18:489–498
Palmisano AC, Sullivan CW (1983) Physiology of sea ice diatoms. II. Dark survival of three polar diatoms. Can J Microbiol 29:157–160
Parsons TR, Maita Y, Lalli CM (1984) Photosynthesis as measured by the uptake of radioactive carbon. In: Lalli TR (ed) A manual of chemical & biological methods for seawater analysis. Pergamon, Amsterdam, pp 115–120
Peters E (1996) Prolonged darkness and diatom mortality 2. Marine temperate species. J Exp Mar Biol Ecol 207:43–58
Peters E, Thomas DN (1996) Prolonged darkness and diatom mortality I: marine Antarctic species. J Exp Mar Biol Ecol 207:25–41
Petrou K, Ralph P (2011) Photosynthesis and net primary productivity in three Antarctic diatoms: possible significance for their distribution in the Antarctic marine ecosystem. Mar Ecol Prog Ser 437:27–40
Petrou K, Hill R, Brown CM, Campbell DA, Doblin MA, Ralph PJ (2010) Rapid photoprotection in sea-ice diatoms from the East Antarctic pack ice. Limnol Oceanogr 55:8
Petrou K, Doblin M, Ralph P (2011) Heterogeneity in the photoprotective capacity of three Antarctic diatoms during short-term changes in salinity and temperature. Mar Biol 158:1029–1041
Petrou K, Kranz SA, Trimborn S, Hassler CS, Ameijeiras SB, Sackett O, Ralph PJ, Davidson AT (2016) Southern Ocean phytoplankton physiology in a changing climate. J Plant Physiol 203:135–150
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
Popels LC, Hutchins DA (2002) Factors affecting dark survival of the brown tide alga Aureococcus anophagefferens (Pelagophyceae). J Phycol 38:738–744
Poulin M, Daugbjerg N, Gradinger R, Ilyash L, Ratkova T, von Quillfeldt C (2011) The pan-Arctic biodiversity of marine pelagic and sea-ice unicellular eukaryotes: a first-attempt assessment. Mar Biodivers 41:13–28
Reeves S, McMinn A, Martin A (2011) The effect of prolonged darkness on the growth, recovery and survival of Antarctic sea ice diatoms. Polar Biol 34:1019–1032
Rivkin RB, Putt M (1987) Heterotrophy and photoheterotrophy by antarctic microalgae—light-dependent incorporation of amino-acids and glucose. J Phycol 23:442–452
Sakshaug E (2004) Primary and secondary production in Arctic seas. In: Stein ER, Macdonald RW (eds) The organic carbon cycle in the Arctic Ocean. Springer, Berlin, pp 57–81
Schaub I, Wagner H, Graeve M, Karsten U (2017) Effects of prolonged darkness and temperature on the lipid metabolism in the benthic diatom Navicula perminuta from the Arctic Adventfjorden, Svalbard. Polar Biol 40:1425–1439
Schuback N, Schallenberg C, Duckham C, Maldonado MT, Tortell PD (2015) Interacting effects of light and iron availability on the coupling of photosynthetic electron transport and CO2-assimilation in marine phytoplankton. PLoS ONE 10:e0133235
Schuback N, Hoppe CJM, Tremblay J-É, Maldonado MT, Tortell PD (2017) Primary productivity and the coupling of photosynthetic electron transport and carbon fixation in the Arctic Ocean. Limnol Oceanogr 62:898–921
Silsbe GM, Oxborough K, Suggett DJ, Forster RM, Ihnken S, Komárek O, Lawrenz E, Prášil O, Röttgers R, Šicner M, Simis SGH, Van Dijk MA, Kromkamp JC (2015) Toward autonomous measurements of photosynthetic electron transport rates: an evaluation of active fluorescence-based measurements of photochemistry. Limnol Oceanogr 13:138–155
Smayda TJ, Mitchell-Innes B (1974) Dark survival of autotrophic, planktonic marine diatoms. Mar Biol 25:195–202
Smith AE, Morris I (1980) Pathways of carbon assimilation in phytoplankton from the antarctic ocean. Limnol Oceanogr 25:865–872
Suggett D, Moore CM, Geider R (2010) Estimating aquatic productivity from active fluorescence measurements. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 103–127
Thomas SL, Campbell DA (2013) Photophysiology of Bolidomonas pacifica. J Plankton Res 35:260–269
van de Poll WHV, Lagunas M, de Vries T, Visser RJW, Buma AGJ (2011) Non-photochemical quenching of chlorophyll fluorescence and xanthophyll cycle responses after excess PAR and UVR in Chaetoceros brevis, Phaeocystis antarctica and coastal Antarctic phytoplankton. Mar Ecol Prog Ser 426:119–131
Wagner H, Jakob T, Wilhelm C (2006) Balancing the energy flow from captured light to biomass under fluctuating light conditions. New Phytol 169:95–108
White AW (1974) Growth of two facultatively heterotrophic marine centric diatoms. J Phycol 10:292–300
Wu Y, Jeans J, Suggett D, Finkel Z, Campbell DA (2014) Large centric diatoms allocate more cellular nitrogen to photosynthesis to counter slower RUBISCO turnover rates. Front Mar Sci 1:1–11
Wulff A, Roleda MY, Zacher K, Wiencke C (2008) Exposure to sudden light burst after prolonged darkness—a case study on benthic diatoms in antarctica. Diatom Res 23:519–532
Young JN, Goldman JAL, Kranz SA, Tortell PD, Morel FMM (2015) Slow carboxylation of Rubisco constrains the rate of carbon fixation during Antarctic phytoplankton blooms. New Phytol 205:172–181
Zapata M, Rodriguez F, Garrido JL (2000) Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar Ecol Prog Ser 195:29–45
Zhu Y, Ishizaka J, Tripathy SC, Wang S, Sukigara C, Goes J, Matsuno T, Suggett DJ (2017) Relationship between light, community composition and the electron requirement for carbon fixation in natural phytoplankton. Mar Ecol Prog Ser 580:83–100
Acknowledgements
We thank a joint contribution to the research programs of UMI Takuvik, ArcticNet (Network Centres of Excellence of Canada), the Canada Excellence Research Chair in Remote Sensing of Canada’s New Arctic Frontier, and the Canada Research Chair program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lacour, T., Morin, PI., Sciandra, T. et al. Decoupling light harvesting, electron transport and carbon fixation during prolonged darkness supports rapid recovery upon re-illumination in the Arctic diatom Chaetoceros neogracilis. Polar Biol 42, 1787–1799 (2019). https://doi.org/10.1007/s00300-019-02507-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-019-02507-2