Abstract
Key message
The Arabidopsis mutant ( ucu2 - 2/gi - 2 ) is thaxtomin A, isoxaben and NPA-sensitive indicated by root growth and ion flux responses providing new insights into these compounds mode of action and interactions.
Abstract
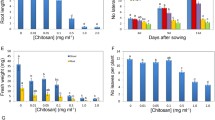
Thaxtomin A (TA) is a cellulose biosynthetic inhibitor (CBI) that promotes plant cell hypertrophy and cell death. Electrophysiological analysis of steady-state K+ and Ca2+ fluxes in Arabidopsis thaliana roots pretreated with TA for 24 h indicated a disturbance in the regulation of ion movement across the plant cell membrane. The observed inability to control solute movement, recorded in rapidly growing meristematic and elongation root zones, may partly explain typical root toxicity responses to TA treatment. Of note, the TA-sensitive mutant (ucu2-2/gi-2) was more susceptible with K+ and Ca2+ fluxes altered between 1.3 and eightfold compared to the wild-type control where fluxes altered between 1.2 and threefold. Root growth inhibition assays showed that the ucu2-2/gi-2 mutant had an increased sensitivity to the auxin 2,4-D, but not IAA or NAA; it also had increased sensitivity to the auxin efflux transport inhibitor, 1-naphthylphthalamic acid (NPA), but not 2,3,5- Triiodobenzoic acid (TIBA), when compared to the WT. The NPA sensitivity data were supported by electrophysiological analysis of H+ fluxes in the mature (but not elongation) root zone. Increased sensitivity to the CBI, isoxaben (IXB), but not dichlobenil was recorded. Increased sensitivity to both TA and IXB corresponded with higher levels of accumulation of these toxins in the root tissue, compared to the WT. Further root growth inhibition assays showed no altered sensitivity of ucu2-2/gi-2 to two other plant pathogen toxins, alternariol and fusaric acid. Identification of a TA-sensitive Arabidopsis mutant provides further insight into how this CBI toxin interacts with plant cells.




Similar content being viewed by others
References
Anschutz U, Becker D, Shabala S (2014) Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J Plant Physiol 171:670–687
Bailly A, Sovero V, Geisler M (2006) The twisted dwarf’s ABC—how immunophilins regulate auxin transport. Plant Signal Behav 1:277–280
Bailly A, Sovero V, Vincenzetti V et al (2008) Modulation of P-glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins. J Biol Chem 283:21817–21826
Bischoff V, Cookson SJ, Wu S, Scheible WR (2009) Thaxtomin A affects CESA-complex density, expression of cell wall genes, cell wall composition, and causes ectopic lignification in Arabidopsis thaliana seedlings. J Exp Bot 60:955–965
Bouchard R, Bailly A, Blakeslee JJ et al (2006) Immunophilin-like TWISTED DWARF1 modulates auxin efflux activities of Arabidopsis P-glycoproteins. J Biol Chem 281:30603–30612
Brochu V, Girard-Martel M, Duval I et al (2010) Habituation to thaxtomin A in hybrid poplar cell suspensions provides enhanced and durable resistance to inhibitors of cellulose synthesis. BMC Plant Biol 10:272
Cooke T, Poli D, Sztein A, Cohen J (2002) Evolutionary patterns in auxin action. Plant Mol Biol 49:319–338
Demidchik V, Cuin TA, Svistunenko D et al (2010) Arabidopsis root K+ -efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J Cell Sci 123:1468–1479
Demuner AJ, Barbosa LC, Miranda AC et al (2013) The fungal phytotoxin alternariol 9-methyl ether and some of its synthetic analogues inhibit the photosynthetic electron transport chain. J Nat Prod 76:2234–2245
Desprez T, Vernhettes S, Fagard M et al (2002) Resistance against herbicide isoxaben and cellulose deficiency caused by distinct mutations in same cellulose synthase isoform CESA6. Plant Physiol 128:482–490
Duval I, Beaudoin N (2009) Transcriptional profiling in response to inhibition of cellulose synthesis by thaxtomin A and isoxaben in Arabidopsis thaliana suspension cells. Plant Cell Rep 28:811–830
Duval I, Brochu V, Simard M, Beaulieu C, Beaudoin N (2005) Thaxtomin A induces programmed cell death in Arabidopsis thaliana suspension-cultured cells. Planta 222:820–831
Errakhi R, Dauphin A, Meimoun P et al (2008) An early Ca2+ influx is a prerequisite to thaxtomin A-induced cell death in Arabidopsis thaliana cells. J Exp Bot 59:4259–4270
Friml J, Palme K (2002) Polar auxin transport—old questions and new concepts? Plant Mol Biol 49:273–284
Jiao J, Zhou B, Zhu X, Gao Z, Liang Y (2013) Fusaric acid induction of programmed cell death modulated through nitric oxide signalling in tobacco suspension cells. Planta 238:727–737
Kers JA, Cameron KD, Joshi MV et al (2005) A large, mobile pathogenicity island confers plant pathogenicity on Streptomyces species. Mol Microbiol 55:1025–1033
King RR, Lawrence CH, Clark MC, Calhoun LA (1989) Isolation and characterisation of phytotoxins associated with Streptomyces scabies. J Chem Soc Chem Commun 13:849–850
Lawrence CH, Clark MC, King RR (1990) Induction of common scab symptoms in aseptically cultured potato tubers by the vivotoxin, thaxtomin. Phytopathology 80:606–608
Lomax T, Muday G, Rubery P (1995) Auxin transport. In: Davies P (ed) Plant hormones: physiology, biochemistry, and molecular biology, 2nd edn. Kluwer Academic Publishers, Dordrecht, pp 509–530
Loria R, Kers J, Joshi M (2006) Evolution of plant pathogenicity in Streptomyces. Ann Rev Phytopathol 44:469–487
Melida H, Encina A, Alvarez J, Luis Acebes J, Caparros-Ruiz D (2010) Unraveling the biochemical and molecular networks involved in maize cell habituation to the cellulose biosynthesis inhibitor dichlobenil. Mol Plant 3:842–853
Molesworth PP, Gardiner MG, Jones RC, Smith JA, Tegg RS, Wilson C (2010) Synthesis and phytotoxicity of structural analogues of thaxtomin natural products. Aust J Chem 63:813–820
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Nakagawa N, Sakurai N (1998) Increase in the amount of celA1 protein in tobacco BY-2 cells by a cellulose biosynthesis inhibitor, 2,6-dichlorobenzonitrile. Plant Cell Physiol 39:779–785
Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SI, Chen RJ, Masson PH, Murphy AS (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16:1898–1911
Peer WA, Blakeslee JJ, Yang H, Murphy AS (2011) Seven things we think we know about auxin transport. Mol Plant 4:487–504
Perez-Perez JM, Ponce MR, Micol JL (2004) The ULTRACURVATA2 gene of Arabidopsis encodes an FK506-binding protein involved in auxin and brassinosteroid signaling. Plant Physiol 134:101–117
Petrasek J, Friml J (2009) Auxin transport routes in plant development. Development 136:2675–2688
Qi Z, Verma R, Gehring C, Yamaguchi Y, Zhao Y, Ryan CA, Berkowitz GA (2010) Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc Natl Acad Sci 107:21193–21198
Redei GP (1962) Supervital mutants in Arabidopsis. Genetics 47:443–460
Scheible WR, Eshed R, Richmond T, Delmer D, Somerville C (2001) Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis ixr1 mutants. Proc Natl Acad Sci 98:10079–10084
Scheible WR, Fry B, Kochevenko A et al (2003) An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthesis inhibitor from Streptomyces species. Plant Cell 15:1781–1794
Shabala S (2009) Salinity and programmed cell death: unravelling mechanisms for ion specific signalling. J Exp Bot 60:709–711
Shabala L, Ross T, McMeekin T, Shabala S (2006) Non-invasive microelectrode ion flux measurements to study adaptive responses of microorganisms to the environment. FEMS Microbiol Rev 30:472–486
Shabala S, Cuin TA, Prismall L, Nemchinov LG (2007) Expression of animal CED-9 anti-apoptotic gene in tobacco modifies plasma membrane ion fluxes in response to salinity and oxidative stress. Planta 227:189–197
Shapiro AD (2000) Using Arabidopsis mutants to delineate disease resistance signaling pathways. Can J Plant Pathol 22:199–216
Tegg RS, Melian L, Wilson CR, Shabala S (2005) Plant cell growth and ion flux responses to the streptomycete phytotoxin thaxtomin A: calcium and hydrogen flux patterns revealed by the non-invasive MIFE technique. Plant Cell Physiol 46:638–648
Tegg RS, Gill WM, Thompson HK, Davies NW, Ross JJ, Wilson CR (2008) Auxin-induced resistance to common scab disease of potato linked to inhibition of thaxtomin A toxicity. Plant Dis 92:1321–1328
Tegg RS, Corkrey R, Wilson CR (2012) Relationship between the application of foliar chemicals to reduce common scab disease of potato and correlation with thaxtomin A toxicity. Plant Dis 96:97–103
Tegg RS, Shabala SN, Cuin TA, Davies NW, Wilson CR (2013) Enhanced resistance to the cellulose biosynthetic inhibitors, thaxtomin A and isoxaben in Arabidopsis thaliana mutants, also provides specific co-resistance to the auxin transport inhibitor, 1-NPA. BMC Plant Biol 13:76
Wilson CR, Luckman GA, Tegg RS, Yuan ZQ, Wilson AJ, Eyles A, Conner AJ (2009) Enhanced resistance to common scab of potato through somatic cell selection in cv. Iwa with the phytotoxin thaxtomin A. Plant Pathol 58:137–144
Acknowledgments
For assistance with statistical analyses we thank D. Ratkowsky and R. Corkrey. For assistance with quantification of toxins from plant tissues we thank N. Davies. This work was supported by a postgraduate scholarship award from the University of Tasmania (to R.T.) and financial support from Horticulture Australia Limited (HAL) and the potato levy in partnership with the Potato Processing Association of Australia (to C.W.). The Australian Government provides matched funding for all HAL’s R&D activities. The work was also supported by a grant from the Australian Research Council (to S.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by C.-H. Dong.
Rights and permissions
About this article
Cite this article
Tegg, R.S., Shabala, S., Cuin, T.A. et al. Mechanisms of thaxtomin A-induced root toxicity revealed by a thaxtomin A sensitive Arabidopsis mutant (ucu2-2/gi-2). Plant Cell Rep 35, 347–356 (2016). https://doi.org/10.1007/s00299-015-1888-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-015-1888-4