Abstract
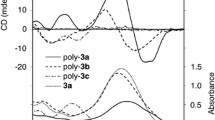
A series of novel N-propargylamides carrying dipole azobenzene chromophores were synthesized and polymerized with [Rh(nbd)Cl]2 catalyst to obtain cis-transoid poly(N-propargylamides). The solubility, thermal stability and conformation of these polymers were also studied. The introduction of valeryl group increased the solubility of this kind of poly(N-propargylamides). All the polymers exhibited a good, thermal stability. CD and UV–Vis spectra showed that poly(N-propargylamide) (Poly(V2b)) took a tight helical structure with predominantly one-handed screw sense. Poly(V2b) exhibited a good stable helical structure at various temperatures (5–60 °C). It also maintained good helicity in polar solvent. The improved helix stability could be attributed to the enhanced hydrogen bonding strength and steric repulsion between the newly designed azobenzene groups. Furthermore, the electrostatic repulsion between the dipole azobenzene chromophores in the side chains improved the helix stability as well. The backbone of Poly(V2b) still kept helical sense after the isomerization of trans-azobenzene to the cis form under UV irradiation.








Similar content being viewed by others
References
Maeda K, Mochizuki H, Osato K, Yashima E (2011) Stimuli-responsive helical poly(phenylacetylene)s bearing cyclodextrin pendants that exhibit enantioselective gelation in response to chirality of a chiral amine and hierarchical super-structured helix formation. Macromolecules 44:3217–3226
Zhu ZG, Cui JX, Zhang J, Wan XH (2012) Hydrogen bonding of helical vinyl polymers containing alanine moieties: a stabilized interaction of helical conformation sensitive to solvents and pH. Polym Chem 3:668–678
Maeda K, Tanaka K, Morino K, Yashima E (2007) Synthesis of optically active helical poly(phenylacetylene)s bearing oligopeptide pendants and their use as polymeric organocatalysts for asymmetric epoxidation. Macromolecules 40:6783–6785
Yamamoto T, Yamada T, Nagata Y, Suginome M (2010) High-molecular-weight polyquinoxaline-based helically chiral phosphine (PQXphos) as chirality-switchable, reusable, and highly enantioselective monodentate ligand in catalytic asymmetric hydrosilylation of styrenes. J Am Chem Soc 132:7899–7901
Ohsawa S, Sakurai S, Nagai K, Maeda K, Kumaki J, Yashima E (2012) Amplification of macromolecular helicity of dynamic helical poly(phenylacetylene)s bearing non-racemic alanine pendants in dilute solution, liquid crystal and two-dimensional crystal. Poylm J 44:42–50
Song C, Li L, Wang FJ, Deng JP, Yang WT (2011) Novel optically active helical poly(N-propargylthiourea)s: synthesis, characterization and complexing ability toward Fe(III) ions. Polym Chem 2:2825–2829
Sanda F, Teraura T, Masuda T (2004) Synthesis of chiral polyacetylenes carrying amino acids and azobenzenes and transformation of the higher order structure by photoirradiation. J Polym Sci, Part A: Polym Chem 42:4641–4647
Sogawa H, Shiotsuki M, Matsuoka H, Sanda F (2011) Synthesis, chiroptical properties, and photoresponsiveness of optically active poly(m-phenyleneethynylene)s containing azobenzene moieties. Macromolecules 44:3338–3345
Schwartz E, Koepf M, Kitto HJ, Nolte RJM, Rowan AE (2011) Helical poly(isocyanides): past, present and future. Polym Chem 2:33–47
Nakano T, Okamoto Y (2001) Synthetic helical polymers: conformation and function. Chem Rev 101:4013–4038
Okamoto Y, Suzuki K, Ohta K, Hatada K, Yuki H (1979) Optically active poly(triphenylmethyl methacrylate) with one-handed helical conformation. J Am Chem Soc 101:4763–4765
Lu H, Wang J, Bai YG, Lang JW, Liu SY, Lin Y, Chen JJ (2011) Ionic polypeptides with unusual helical stability. Nat Commun 2:206
Gabrielson NP, Lu H, Yin LC, Li D, Wang F, Cheng JJ (2012) Reactive and bioactive cationic α-helical polypeptide template for nonviral gene delivery. Angew Chem Int Ed 124:1169–1173
Shiotsuki M, Sanda F, Masuda T (2011) Polymerization of substituted acetylenes and features of the formed polymers. Polym Chem 2:1044–1058
Liu JZ, Lam JWY, Tang BZ (2009) Acetylenic polymers: syntheses, structures, and functions. Chem Rev 109:5799–5867
Yashima E, Maeda K, Sato O (2001) Switching of a macromolecular helicity for visual distinction of molecular recognition events. J Am Chem Soc 123:8159–8160
Cheuk KKL, Lam JWY, Chen J, Lai LM, Tang BZ (2003) Amino acid-containing polyacetylenes: synthesis, hydrogen bonding, chirality transcription, and chain helicity of amphiphilic poly(phenylacetylene)s carrying l-leucine pendants. Macromolecules 36:5947–5959
Cheuk KKL, Lam JWY, Lai LM, Dong YP, Tang BZ (2003) Syntheses, hydrogen-bonding interactions, tunable chain helicities, and cooperative supramolecular associations and dissociations of poly(phenylacetylene)s bearing l-valine pendants: toward the development of proteomimetic polyenes. Macromolecules 36:9752–9762
Li BS, Cheuk KKL, Salhi F, Lam JWY, Cha JAK, Xiao XD, Bai CL, Tang BZ (2001) Tuning the chain helicity and organizational morphology of an l-valine-containing polyacetylene by pH change. Nano Lett 1:323–328
Nomura R, Tabei J, Nishiura S, Masuda T (2003) A helix in helices: a helical conjugated polymer that has helically arranged hydrogen-bond strands. Macromolecules 36:561–564
Nomura R, Tabei J, Masuda T (2001) Biomimetic stabilization of helical structure in a synthetic polymer by means of intramolecular hydrogen bonds. J Am Chem Soc 123:8430–8431
Nomura R, Tabei J, Masuda T (2002) Effect of side chain structure on the conformation of poly(N-propargylalkylamide). Macromolecules 35:2955–2961
Tabei J, Nomura R, Masuda T (2002) Conformational study of poly(N-propargylamides) having bulky pendant groups. Macromolecules 35:5405–5409
Bandara HMD, Burdette SC (2012) Photoisomerization in different classes of azobenzene. Chem Soc Rev 41:1809–1825
Wu WB, Huang LJ, Song CF, Yu G, Ye C, Liu YQ, Qin JG, Li QQ, Li Z (2012) Novel global-like second-order nonlinear optical dendrimers: convenient synthesis through powerful click chemistry and large NLO effects achieved by using simple azo chromophore. Chem Sci 3:1256–1261
Lee MJ, Jung DH, Han YK (2006) Photo-responsive polymers and their applications to optical memory. Mol Cryst Liq Cryst 444:41–50
Camorani P, Fontana MP (2006) Optical control of structural morphology in azobenzene containing polymeric liquid crystals. Phy Rev E 73:011703
Fujii T, Shiotsuki M, Inai Y, Sanda F, Masuda T (2007) Synthesis of helical poly(N-propargylamides) carrying azobenzene moieties in side chains. Reversible arrangement-disarrangement of helical side chain arrays upon photoirradiation keeping helical main chain intact. Macromolecules 40:7079–7088
Zhou JL, Chen XF, Fan XH, Lu CX, Zhou QF (2006) Synthesis and chiroptical properties of optically active poly(N-propargylamide) bearing photoisomerizable azobenzene moieties. J Polym Sci, Part A: Polym Chem 44:6047–6054
Zhao HC, Sanda F, Masuda T (2006) Stimuli-responsive conjugated polymers. synthesis and chiroptical properties of polyacetylene carrying l-glutamic acid and azobenzene in the side chain. Polymer 47:2596–2602
Robinson BH, Dalton LR (2000) Monte Carlo statistical mechanical simulations of the competition of intermolecular electrostatic and poling-field interactions in defining macroscopic electro-optic activity for organic chromophore/polymer materials. J Phys Chem A 104:4785–4795
Ma H, Chen BQ, Sassa T, Dalton LR, Jen AK-Y (2001) Highly efficient and thermally stable nonlinear optical dendrimer for electrooptics. J Am Chem Soc 123:986–987
Kishimoto Y, Itou M, Miyatake T, Ikariya T, Noyori R (1995) Polymerization of monosubstituted acetylenes with a zwitterionic Rhodium(I) complex, Rh+(2,5-norbornadiene)[η6-C6H5)B-(C6H5)3. Macromolecules 28:6662–6666
Sanda F, Tabei J, Shiotsuki M, Masuda T (2006) Chiroptical study and conformation analysis of helical polymers surrounded by helical hydrogen-bonding strands. Sci Tech Adv Mater 7:572–577
Gao G, Sanda F, Masuda T (2003) Synthesis and properties of amino acid-based polyacetylenes. Macromolecules 36:3932–3937
Acknowledgments
The work described in this article was supported by grants from the Shanghai Natural Science Foundation of China (11ZR1410600), the National Natural Science Foundation of China (21204021), and Fundamental Research Funds for the Central Universities of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Ren, X., Li, S. et al. Synthesis and conformational study of novel, stable, helical poly(N-propargylamides) containing dipole azobenzene chromophores in the side chains. Polym. Bull. 71, 2803–2818 (2014). https://doi.org/10.1007/s00289-014-1223-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1223-1