Abstract
Objectives
AUY922 is a novel heat shock protein inhibitor with preclinical activity in pancreatic cancer. This phase II study evaluated the efficacy of AUY922 in patients with advanced pancreatic cancer previously treated with chemotherapy.
Methods
In this single-arm, Simon two-stage phase II trial, patients with metastatic or locally advanced pancreatic ductal adenocarcinoma who had progressed on at least one line of chemotherapy and were of good performances status (ECOG 0 or 1) were treated with AUY922 at a dose of 70 mg/m2 IV weekly. The primary endpoint was disease control rate (objective response and stable disease ≥16 weeks).
Results
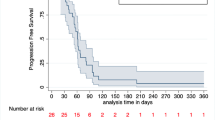
Twelve patients were accrued, all of whom received treatment. At least possibly related ≥grade 3 adverse events included fatigue (8 %) and AST elevation (8 %). Ten patients were evaluable for response with 1 (10 %) having stable disease and 9 (90 %) progressive disease. The median progression-free survival was 1.6 months, and the median overall survival was 2.9 months.
Conclusions
AUY922 was not associated with significant efficacy in previously treated patients with advanced pancreatic cancer.


Similar content being viewed by others
References
Rahib L et al (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74:2913–2921
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Von Hoff DD et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703
Kulke MH, Blaszkowsky LS, Ryan DP et al (2007) Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol 25:4787–4792
Cantore M, Rabbi C, Fiorentini G et al (2004) Combined irinotecan and oxaliplatin in patients with advanced pre-treated pancreatic cancer. Oncology 67:93–97
Demols A, Peeters M, Polus M et al (2006) Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: a phase II study. Br J Cancer 94:481–485
Xiong HQ, Varadhachary GR, Blais JC et al (2008) Phase 2 trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer 113:2046–2052
Sudo K, Yamaguchi T, Nakamura K et al (2011) Phase II study of S-1 in patients with gemcitabine-resistant advanced pancreatic cancer. Cancer Chemother Pharmacol 67:249–254
Wolpin BM, Hezel AF, Abrams T et al (2009) Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol 27:193–198
Hosein PJ, de Lima Lopes G Jr, Pastorini VH et al (2013) A phase II trial of nab-Paclitaxel as second-line therapy in patients with advanced pancreatic cancer. Am J Clin Oncol 36(2):141–156
Pelzer U, Kubica K, Stieler J et al (2008) A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO 003 study. J Clin Oncol 26:4508
Gill S, Ko YJ, Cripps C et al (2014) PANCREOX: a randomized phase 3 study of 5FU/LV with or without oxaliplatin for second-line advanced pancreatic cancer (APC) in patients (pts) who have received gemcitabine (GEM)-based chemotherapy (CT). J Clin Oncol 32(5s):4022
Renouf DJ et al (2014) A phase II study of erlotinib in gemcitabine refractory advanced pancreatic cancer. Eur J Cancer (Oxf Engl 1990) 50:1909–1915
Pearl LH, Prodromou C (2006) Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem 71:271–294
Pratt W, Toft D (2003) Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med 228:111–133
Kamal A, Thao L, Sensintaffar J et al (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425:407–410
Novartis AUY922 Investigator’s Brochure, July 2010
Erlichmann C (2009) Tanespimycin: the opportunities and challenges of targeting heat shock protein 90. Expert Opin Investig Drugs 18(6):861–868
Jensen M, Schoepfer J, Radimerski T et al (2008) NVP-AUY922: a small molecule HSP90 inhibitor with potent antitumor activity in preclinical breast cancer models. Breast Cancer Res 10(2):R33
Moser C, Lang SA, Hackl C et al (2012) Targeting HSP90 by the novel inhibitor NVP-AUY922 reduces growth and angiogenesis of pancreatic cancer. Anticancer Res 32(7):2551–2561
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst 92:205–216
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Infante JR, Somer BG, Park JO et al (2014) A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer 50(12):2072–2081
Ko AH, Bekaii-Saab T, Van Ziffle J et al (2016) A multicenter, open-label phase II clinical trial of combined MEK plus EGFR inhibition for chemotherapy-refractory advanced pancreatic adenocarcinoma. Clin Cancer Res 22(1):61–68
Kaufman B, Shapira-Frommer R, Schmutzler RK et al (2015) Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 33(3):244–250
Holter S, Borgida A, Dodd A et al (2015) Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol 33(28):3124–3129
Le DT, Wang-Gillam A, Picozzi V et al (2015) Safety and survival with GVAX pancreas prime and listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 33(12):1325–1333
Phase II study of MEDI4736 monotherapy or in combinations with tremelimumab in metastatic pancreatic ductal carcinoma. NCT02558894
Le DT, Uram JN, Wang H et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372(26):2509–2520
Riazy M, Kalloger S, Sheffeld B et al. (2015) Mismatch repair status may predict response to adjuvant chemotherapy in resectable pancreatic ductal adenocarcinoma. Modern Pathol 28(10): 1383–1389
Waddell N et al (2015) Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518:495–501
Sausen M, Phallen J, Adleff V et al (2015) Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat Commun 7(6):7686
Moffitt RA, Marayati R, Flate EL et al (2015) Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 47(10):1168–1178
Bailey P, Chang DK, Nones K et al. (2016) Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531(7592):47–52
Acknowledgments
This study was funded by a grant from Novartis. Novartis did not contribute to the data analysis, interpretation, or manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no relevant conflicts of interest.
Rights and permissions
About this article
Cite this article
Renouf, D., Hedley, D., Krzyzanowska, M.K. et al. A phase II study of the HSP90 inhibitor AUY922 in chemotherapy refractory advanced pancreatic cancer. Cancer Chemother Pharmacol 78, 541–545 (2016). https://doi.org/10.1007/s00280-016-3102-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3102-y