Abstract
Purpose
To evaluate the postprocedural imaging findings and safety of repeated intra-arterial therapy via the cystic artery in patients with hepatocellular carcinoma (HCC).
Methods
This retrospective study was approved by our institutional review board. From February 2002 to January 2012, we performed repeated (two or more) chemotherapeutic infusion or chemoembolization via the cystic artery using iodized oil in 132 patients with HCCs. Computed tomographic (CT) scans, digital subtraction angiograms, and medical records were retrospectively reviewed by consensus.
Results
A total of 340 sessions of intra-arterial therapy (160 sessions of chemotherapeutic infusion and 180 sessions of chemoembolization) via the cystic artery were undertaken in 132 patients. Fifty-five of 132 patients received both chemotherapeutic infusion and chemoembolization. The incidence of gallbladder wall thickening on follow-up contrast-enhanced CT was significantly higher in chemoembolization (48 of 180, 26.7 %) than in chemotherapeutic infusion (27 of 160, 16.9 %) (P = 0.035). Persistent gallbladder wall thickening was more frequently observed in chemoembolization (48 of 107, 44.9 %) than in chemotherapeutic infusion (27 of 90, 30 %) (P = 0.039). The major complication rate was 15 of 340 sessions (4.4 %) with 11 of 132 patients (8.3 %). Acute cholecystitis, which was related to intra-arterial therapy via the cystic artery, developed in two patients and was managed by conservative treatment.
Conclusion
HCC supplied by the cystic artery can be safely treated by repeated intra-arterial chemotherapeutic infusion or chemoembolization using iodized oil through the cystic artery.
Similar content being viewed by others
Introduction
The cystic artery arises from the first branch of the main right hepatic artery and subsequently divides into the deep and superficial branches. The cystic artery usually supplies the liver parenchyma near the gallbladder bed as well as the gallbladder [1, 2]. The clinical significance of the cystic artery during chemoembolization is the possibility of ischemic complications of the gallbladder [3–12]. The incidence and outcomes of cholecystitis after transcatheter arterial chemoembolization (TACE) is somewhat controversial. Hirota et al. [13] suggested that it was impossible to embolize the cystic artery when it fed hepatocellular carcinoma (HCC) and that the HCC should be treated with surgical resection or percutaneous alcohol injection. However, the development of small-caliber microcatheter systems has made it possible to perform highly selective chemoembolization, leading to safer embolization of the cystic artery than occurred several decades ago. Kang et al. [14] described 27 patients with HCC that were supplied exclusively by the cystic artery and underwent chemotherapeutic infusion or chemoembolization using iodized oil. All of the patients were successfully treated with no major complications, including acute ischemic cholecystitis or gallbladder perforation.
During the past 10 years, we have attempted repeated chemotherapeutic infusion or chemoembolization through the cystic artery in patients with HCCs. To our knowledge, there has been no report regarding the safety of repeated chemotherapeutic infusion or chemoembolization via the cystic artery for the treatment of HCC. We evaluated the postprocedural imaging findings and the safety of repeated chemotherapeutic infusion or chemoembolization via the cystic artery in patients with HCC.
Patients and Methods
This retrospective study was approved by the institutional review board of our hospital.
Patients
Between February 2002 and January 2012, a total of 5,902 patients with HCC received 24,146 sessions of chemoembolization at our institution. Among these patients, 433 (7.3 %) with HCC had undergone at least one session of chemotherapeutic infusion or chemoembolization through the cystic artery. The inclusion criteria were as follows: (1) a confirmative diagnosis of HCC based on clinical or laboratory test findings (e.g., elevated α-fetoprotein levels and viral markers) in combination with typical computed tomographic (CT) and angiographic findings; (2) intra-arterial therapy via the cystic artery in two or more sessions; (3) adequate angiographic findings, demonstrating the entire cystic artery anatomy and tumors fed by the cystic artery; (4) baseline CT performed with contrast enhancement before the interventional procedure; and (5) follow-up unenhanced CT scan (mean 14.7 days, range 7–17 days) and contrast-enhanced CT scan (mean 63.7 days, range 22–156 days) after the interventional procedure. We excluded 301 patients as a result of the following conditions: (1) only a single session of the intra-arterial therapy via the cystic artery (n = 293); (2) no available angiographic or CT images (n = 7); and (3) previous medical history of cholecystectomy (n = 1). As a result, a total of 132 patients (112 men and 20 women; mean age 59.7 years; age range 18–86 years) were included in this study.
Chemoembolization Technique
Contrast-enhanced CT was performed in all patients within 30 days (mean, 15 days) prior to chemoembolization with various multidetector-row CT scanners. All chemoembolization procedures were performed by one of three experienced interventional radiologists using standard methods. Celiac angiography was conducted using a 5F Rösch hepatic catheter (Cook, Bloomington, IN). For selective angiography, a microcatheter with a 2.4 F tip (Microferret; Cook) or 2.0F tip (Progreat; Terumo, Tokyo, Japan) was inserted in a coaxial direction.
When selective catheterization was achieved by placement of a microcatheter as close as possible to a specific branch or branches supplying a tumor, iodized oil (Lipiodol; Andre Guerbe, Aulnay-sous-Bios, France) and doxorubicin hydrochloride (Adriamycin RDF; Ildong Pharmaceutical, Seoul, Korea) emulsion was infused until slowing of the antegrade blood flow to the tumor was identified under fluoroscopy. Additional embolization was performed using absorbable gelatin sponge particles measuring 1 mm in diameter (Gelfoam; Upjohn, Kalamazoo, MI, or Cutanplast; Mascia Brunelli, Milano, Italy) soaked in a mixture of 10 mg of doxorubicin hydrochloride and 10 mL of nonionic contrast medium. Chemotherapeutic infusion was defined as injection of iodized oil and doxorubicin emulsion without embolization. Chemoembolization was defined as the infusion of a mixture of doxorubicin and iodized oil followed by embolization with gelatin sponge particle [15]. The indication of gelatin sponge particle embolization was not established. When dense accumulation of iodized oil in the tumor was noted and the cystic artery was relatively small, chemotherapeutic infusion was preferred. When catheterization of the tumor-feeding vessel or deep cystic branch was achieved and the superficial branch was relatively large, chemoembolization was preferred. When we performed gelatin sponge particle embolization, we tried to avoid complete occlusion of the cystic artery. During each session, we infused the chemotherapeutic agent (up to 12 mL of iodized oil and 60 mg of doxorubicin in hydrochloride) via the hepatic artery, the cystic artery and/or extrahepatic collateral arteries.
Data Analysis
CT and digital subtraction angiographic findings were retrospectively reviewed by two of the authors (HHC and HCK), and differences in interpretation were resolved by consensus. On the basis of the angiographic findings, tumors were classified as one of three types, single nodular, multinodular, and infiltrative. We defined the treatment level of the chemotherapeutic infusion or chemoembolization via the cystic artery as follows: (1) tumor feeding vessel, (2) deep branch, (3) superficial branch, or (4) main cystic artery. The degree of iodized oil deposit in the gallbladder wall on postprocedure spot image was classified on the basis of whether the deposit circumscribed the cystic wall more or less than 50 %. On the basis of CT criteria for acute cholecystitis [16–18], the development of gallbladder wall thickening, compared with baseline contrast-enhanced CT, was recorded. The development of gallbladder wall thickening was classified in one of two types. Transient gallbladder wall thickening was defined as gallbladder wall thickening only observed on postprocedural unenhanced CT and restored on follow-up contrast-enhanced CT. Lasting gallbladder wall thickening on both unenhanced CT and follow-up contrast-enhanced CT was defined as persistent gallbladder wall thickening.
Patients’ electronic medical records were reviewed for the determination of complications associated with chemotherapeutic infusion or chemoembolization. Procedure-related complications were classified according to the reporting standards of the Society of Interventional Radiology [19]. Minor complications resulted in no consequences and requiring no therapy (class A) or nominal therapy, including overnight admission for observation only (class B). Major complications required therapy and resulted in minor hospitalization (class C), major hospitalization (class D), permanent adverse sequelae (class E), or death (class F).
The following laboratory values were collected for supplement parameters of cholecystitis as they are established parameters for cholecystitis: white blood cell (WBC) counts, alkaline phosphatase (ALP) levels, and total bilirubin levels [20].
All statistical analyses were performed with SPSS software (version 18.0 for Windows; SPSS, Chicago, IL). The results with P values of less than 0.05 were considered statistically significant. Fisher’s exact test or the Chi square test was used to evaluate the relationship between the postprocedural imaging findings and the type and treatment level of the intra-arterial therapy through the cystic artery.
Results
Ten (7.5 %) patients exhibited single nodular tumors, 101 (76.5 %) patients exhibited multinodular tumors, and 21 patients (16 %) exhibited infiltrative tumors. Intra-arterial therapy via the cystic artery was performed in two sessions (n = 81), three sessions (n = 36), four sessions (n = 10), five sessions (n = 2), six sessions (n = 2), or eight sessions (n = 1). A total of 340 sessions of intra-arterial therapy via the cystic artery were undertaken in 132 patients. Chemotherapeutic infusion was performed in 160 of 340 (47 %) sessions with the following levels: tumor feeding vessel (n = 25), deep branch (n = 21), superficial branch (n = 3), and main cystic artery (n = 111). Chemoembolization was performed in 180 of 340 (53 %) sessions with the following levels: tumor feeding vessel (n = 60), deep branch (n = 47), superficial branch (n = 1), and main cystic artery (n = 72) (Table 1). Fifty-five of 132 patients received both chemotherapeutic infusion and chemoembolization.
The iodized oil deposit circumscribing more than 50 % of the gallbladder wall was noted in 85 of 160 (53 %) sessions after the chemotherapeutic infusion and in 113 of 180 (63 %) sessions after the chemoembolization; results were not statistically significantly different (P = 0.078).
The incidence of gallbladder wall thickening on follow-up contrast-enhanced CT was significantly higher in chemoembolization (48 of 180, 26.7 %) than in chemotherapeutic infusion (27 of 160, 16.9 %) (P = 0.035) (Table 2). Transient gallbladder wall thickening on follow-up CT was the dominant finding in both chemotherapeutic infusion and chemoembolization (Fig. 1), but persistent gallbladder wall thickening was more frequently observed in chemoembolization (48 of 107, 44.9 %) than in chemotherapeutic infusion (27 of 90, 30 %) (P = 0.039).
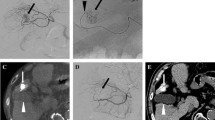
Transient gallbladder wall thickening on follow-up CT after 2 sessions of chemoembolization via the cystic artery in a 50-year-old man with HCC. A Pretreatment baseline contrast-enhanced CT scan demonstrates a normal gallbladder before the procedure (arrow). B Selective angiography of the cystic artery reveals ill-defined tumor staining at superior aspect of the gallbladder (arrow). Note the tip of the microcatheter (arrowhead) within the deep branch of the cystic artery. C Chemoembolization was performed through the deep branch of the cystic artery (open arrow). Spot image during chemoembolization identifies the accumulation of iodized oil in the tumor (solid arrow) and gallbladder wall (arrowheads). D Unenhanced CT scan obtained 2 weeks after chemoembolization demonstrates diffuse gallbladder wall thickening (arrow). E Contrast-enhanced CT 3 months later reveals a normal-appearing gallbladder with complete resolution of the previously noted wall thickening (arrow)
The distribution of gallbladder wall thickening on follow-up CT by the level of therapy in both chemotherapeutic infusion and chemoembolization are summarized in Table 3. The frequency of gallbladder wall thickening on follow-up CT, either transient or persistent, was significantly higher when the intra-arterial therapy was performed through the main cystic artery than through the any branch of the cystic artery in both the chemotherapeutic infusion and chemoembolization groups (P < 0.001, respectively).
Overall, the treatment was well tolerated, and the majority of the patients experienced minor class A complications, including transient right upper quadrant pain, intermittent fever, and nausea with vomiting. Procedure-related major class D complications were developed in 15 of 340 sessions (4.4 %); 10 patients experienced one major complication each, whereas one patient experienced five major complications (Table 4). These patients were hospitalized for 5–45 days after the procedure (mean 14.7 days) and received proper management for the complication.
Of the two cases of acute cholecystitis after intra-arterial therapy via the cystic artery, one patient received two sessions of chemoembolization through the main cystic artery, and the other received four sessions of chemotherapeutic infusion through the main cystic artery. Clinically, these patients complained of abdominal discomfort in the right upper quadrant and were positive for Murphy sign at physical examination 2 days after the procedure. The laboratory findings were consistent with cholecystitis, including WBC counts of 10.1 × 109/L and 14.5 × 109/L, liver enzyme ALP levels of 14 and 163 IU/L, and total bilirubin levels of 1.4 and 2.1 mg/dL, respectively. Both of the patients exhibited iodized oil deposits circumscribing more than 50 % of the gallbladder wall on postprocedural spot image and transient gallbladder wall thickening on follow-up CT (Fig. 2). Conservative treatment with empirical antibiotics was sufficient for the management of acute cholecystitis.
Development of acute cholecystitis after 4 sessions of chemotherapeutic infusion via the cystic artery in a 70-year-old woman with HCC. A Pretreatment baseline contrast-enhanced CT scan demonstrates a collapsed gallbladder without significant wall thickening (arrow). B Common hepatic arteriography reveals multiple nodular tumor stainings. Two nodular tumor stainings (arrows) were supplied by the hypertrophied cystic artery (arrowhead). C Spot image obtained during chemotherapeutic infusion reveals the accumulation of iodized oil in the tumor (arrows) and gallbladder wall (arrowheads). D Postprocedural spot image demonstrated the accumulation of iodized oil in the tumor (arrow) and gallbladder wall (arrowheads). E Unenhanced CT scan obtained 2 weeks after chemotherapeutic infusion demonstrates diffuse gallbladder wall thickening (arrow). F Contrast-enhanced CT 2 months later demonstrates the near-complete recovery of the previously noted gallbladder wall thickening (arrow)
Discussion
Our current results suggest that transient gallbladder wall thickening on follow-up CT is the dominant finding after intra-arterial therapy via the cystic artery for HCC and that persistent gallbladder wall thickening is more frequently observed in chemoembolization than in chemotherapeutic infusion. In addition, intra-arterial therapy through the main cystic artery was the main cause of gallbladder wall thickening. However, intra-arterial therapy with iodized oil via the cystic artery is safe and rarely causes symptomatic cholecystitis, even in repeated treatments.
Wagnetz et al. [10] reported that the imaging findings of acute ischemic cholecystitis developed in 12 of 246 (4.9 %) patients who underwent TACE procedures for HCC. In their cohort, 11 of 12 patients exhibited transient deposits of iodized oil in the gallbladder wall and transient gallbladder wall thickening up to 12 mm on follow-up unenhanced CT. Among these patients, nine of 12 patients had undergone lobar right hepatic artery embolization, which carries the highest risk of inadvertent embolization of the cystic artery.
Similarly, in our study, two patients with acute cholecystitis after the procedure exhibited transient gallbladder wall thickening on follow-up unenhanced CT, possibly suggesting that gallbladder edema is a temporary process but that infarction or necrosis of the gallbladder is rarely results after embolization of the cystic artery using iodized oil. This result can be explained by the difference in the accumulation of iodized oil in the tumor and gallbladder; accumulation is permanent within the tumor but transient with delayed washout in the gallbladder. The frequency of gallbladder wall thickening on follow-up CT is highly associated with the treatment level of intra-arterial therapy via the cystic artery; chemotherapeutic infusion or embolization through the main cystic artery is crucial in both the unintentional technique during the therapy through the lobar or segmental hepatic artery as well as in the selective technique through the cystic artery.
Gallbladder wall edema caused by portal hypertension exhibits thickening of perimuscular connective tissue with water-like attenuation and smooth contour. In contrast, the mechanisms of gallbladder wall thickening by intra-arterial treatment are cytotoxicity, hypoxia, and ischemia resulting thickened muscular layer of the gallbladder with pericholecystic infiltrations. However, it is difficult to differentiate between physiologic gallbladder wall edema and intra-arterial treatment induced gallbladder wall thickening on the basis of the radiologic findings. Correlation of the clinical presentation and chronological imaging findings is thus crucial for determining the cause of the gallbladder wall thickening.
Previous studies [9, 14] have demonstrated the safety of chemoembolization or chemotherapeutic infusion for HCC supplied by the cystic artery exclusively. Miyayama et al. [9] reported that chemoembolization via the tumor feeding vessels from the cystic artery was safe and technically possible in 67 % of patients without severe complications. Kang et al. [14] performed chemotherapeutic infusion or chemoembolization in 27 patients with HCC supplied by the cystic artery exclusively. Chemotherapeutic infusion was performed in 18 patients (67 %), and chemoembolization was performed in nine patients (33 %). The study reported only two minor class A complications, including vasovagal syncope and nausea with vomiting. The authors used a gelatin sponge particle in 33 % of the patients. Gelatin sponge particles were used only for slowing the blood flow of the cystic artery without complete stasis. The authors insisted that this practice contributed to a low complication rate after the procedure.
In our study, we performed repeated intra-arterial therapy via the cystic artery in 132 patients with HCCs over 340 sessions; 160 of 340 (47 %) were performed using chemotherapeutic infusion, and 180 of 340 (53 %) were performed using chemoembolization. We experienced major complications in 15 of 340 (4.4 %) sessions, including four cases of liver abscess, three cases of aggravation of hepatic dysfunction, two cases of acute cholecystitis, two cases of acute renal failure, and one case each of biloma, bile duct stricture, shock, and paralytic ileus. Acute cholecystitis developed in two of 340 (0.6 %) sessions after the procedure; one patient had received chemotherapeutic infusion, and the other had received chemoembolization. Both of the patients had been treated by two sessions of intra-arterial therapy through the main cystic artery. Acute cholecystitis did not develop after the procedure when treating by any branch of the cystic artery. We believed that the incidence of acute cholecystitis is highly associated with the treatment level of the intra-arterial therapy via the cystic artery; intra-arterial therapy via the main cystic artery is one of the major risk factor for the development of acute cholecystitis after the procedure. We also used the gelatin sponge particle to slow the blood flow of the cystic artery without complete stasis. When the gelatin sponge was applied in this way, the use of the gelatin sponge was not a significant risk factor for the development of acute cholecystitis after the procedure.
We frequently encountered the hypertrophied cystic artery supplying the tumor, closely related with the tumor burden. The degree of the cystic artery hypertrophy affected the feasibility of selective catheterization of the tumor feeding vessel or deep cystic branch and might determine the use of gelatin sponge particle and the treatment level of the intra-arterial therapy via the cystic artery.
Ischemic necrosis of the gallbladder is one of the severe potential complications of intra-arterial therapy for hepatic malignancy. This complication is caused by unavoidable embolization of the cystic artery during hepatic artery embolization when the catheter tip is inappropriately positioned beyond the cystic artery [3–6]. Furthermore, the ischemic complication of the gallbladder is more crucial for patients with HCCs fed by the cystic artery [13, 14]. We believe that chemotherapeutic infusion or chemoembolization through the main cystic artery with complete stasis is the main cause of cholecystitis or gallbladder infarction. Because chemoembolization using iodized oil via the cystic artery is relatively safe, as seen in this study, unless the main cystic artery was completely embolized, we suggest that chemotherapeutic infusion or chemoembolization be performed, regardless of the success or failure for selective catheterization of the tumor-feeding vessel of the cystic artery.
Recently, chemoembolization using drug-eluting beads and radioembolization using yttrium-90 have been demonstrated to be efficient treatment options in patients with advanced HCC by outstanding locoregional effects, but drug-eluting beads and radioactive beads exhibit a higher potential risk of cholecystitis and biliary complications compared with conventional TACE [21–24]. Malagari et al. [21] reported that the incidence of cholecystitis was 2.95–5.06 % after TACE for HCC using drug-eluting beads. The authors found that cholecystitis was observed more frequently in segmental embolizations compared with those of subsegmental distribution considered to be the result of inadvertent embolization of the cystic artery. We believe that embolization using drug-eluting beads or radioactive beads via the cystic artery exhibits a higher risk of cholecystitis or gallbladder infarction compared with conventional TACE. With respect to cost–benefit analysis, conventional intra-arterial therapy using iodized oil is more easily available and is cheaper than drug-eluting beads or radioactive beads. Thus, when HCC is supplied by the cystic artery, conventional chemotherapeutic infusion or chemoembolization using iodized oil can be a safe treatment option and may be applied in repeated sessions of intra-arterial therapy without severe complications.
Apart from the intrinsic limits of any retrospective study, several other limitations of our study should be mentioned. First, follow-up CT scans were taken with relatively wide ranges without a standard interval period (7–17 days on unenhanced CT and 22–156 days on contrast-enhanced CT), although unenhanced CT is commonly undertaken 2 weeks after the procedure and contrast-enhanced CT is usually performed 2 months after the procedure in our institution, so the changes in gallbladder wall thickening may be interpreted with little bias. In cases of long-term follow-up periods between unenhanced CT and contrast-enhanced CT, the persistent gallbladder wall thickening may have been misinterpreted as transient thickening. Even the early changes of gallbladder edema or iodized oil deposits in the gallbladder wall may have been missed. Second, given the uneven distribution of the actual numbers of the repeated intra-arterial therapy in each patient (from two sessions to eight sessions), we could not evaluate the relationship between the numbers of repeated intra-arterial treatment and imaging findings or outcome. Further investigation that includes a large population with an even distribution is warranted to clarify the relationship with stronger statistical power. Third, it may be difficult to discriminate between postembolization syndrome and cholecystitis. In addition, ultrasonography and enhanced CT scan are seldom undertaken during hospitalization after the procedure in our institution. Thus, the prevalence of acute cholecystitis might be underestimated.
In conclusion, our results indicate that HCC supplied by the cystic artery can be safely treated by multisession intra-arterial chemotherapeutic infusion or chemoembolization using iodized oil and gelatin sponge particles through the cystic artery. We frequently encountered transient gallbladder wall thickening on follow-up CT, especially in patients treated by the main cystic artery, which typically resolves during the follow-up period.
References
Polyzonis M, Tsikaras P, Hytiroglou P, Agios A (1989) Further observations on the vascular system of the gallbladder in man. Bull Assoc Anat 73:25–28
Wang X, Shah RP, Maybody M et al (2011) Cystic artery localization with a three-dimensional angiography vessel tracking system compared with conventional two-dimensional angiography. J Vasc Interv Radiol 22:1414–1419
Kuroda C, Iwasaki M, Tanaka T et al (1983) Gallbladder infarction following hepatic transcatheter arterial embolization. Angiographic study. Radiology 149:85–89
Takayasu K, Moriyama N, Muramatsu Y et al (1985) Gallbladder infarction after hepatic artery embolization. AJR Am J Roentgenol 144:135–138
Nakamura H, Kondoh H (1986) Emphysematous cholecystitis: complication of hepatic artery embolization. Cardiovasc Intervent Radiol 9:152–153
Simons R, Sinanan M, Coldwell D (1992) Gangrenous cholecystitis as a complication of hepatic artery embolization: case report. Surgery 112:106–110
Chung JW, Park JH, Han JK et al (1996) Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology 198:33–40
Sakamoto I, Aso N, Nagaoki K et al (1998) Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics 18:605–619
Miyayama S, Matsui O, Nishida H et al (2003) Transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma fed by the cystic artery. J Vasc Interv Radiol 14:1155–1161
Wagnetz U, Jaskolka J, Yang P, Jhaveri KS (2010) Acute ischemic cholecystitis after transarterial chemoembolization of hepatocellular carcinoma: incidence and clinical outcome. J Comput Assist Tomogr 34:348–353
McWilliams JP, Kee ST, Loh CT et al (2011) Prophylactic embolization of the cystic artery before radioembolization: feasibility, safety, and outcomes. Cardiovasc Intervent Radiol 34:786–792
Karaman B, Battal B, Oren NC et al (2012) Acute ischemic cholecystitis after transarterial chemoembolization with drug-eluting beads. Clin Imaging 36:861–864
Hirota S, Matsumoto S, Fukuda T et al (1999) Solitary hepatocellular carcinoma fed by the cystic artery: limitation of transcatheter arterial embolization. Cardiovasc Intervent Radiol 22:206–209
Kang B, Kim HC, Chung JW et al (2013) Safety of chemotherapeutic infusion or chemoembolization for hepatocellular carcinoma supplied exclusively by the cystic artery. Cardiovasc Intervent Radiol 36:1313–1319
Brown DB, Nikolic B, Covey AM et al (2012) Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 23:287–294
Havrilla TR, Reich NE, Seidelmann F et al (1978) Computed tomography of the gallbladder. AJR Am J Roentgenol 130:1059–1067
Mirvis SE, Whitley NO, Miller JW (1987) CT diagnosis of acalculouscholecystitis. J Comput Assist Tomogr 11:83–87
Fidler J, Paulson E, Layfield L (1996) CT evaluation of acute cholecystitis: findings and usefulness in diagnosis. AJR Am J Roentgenol 166:1085–1088
Finder D (2003) Clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202
Greenberger NJ, Paumgartner G (2008) Diseases of gallbladder and bile ducts. In: Fauci AS, Braunwald E, Kasper DL, Hauser S (eds) Harrison’s principles of internal medicine, 17th edn. McGraw-Hill, New York
Malagari K, Pomoni M, Spyridopoulos TN et al (2011) Safety profile of sequential transcatheter chemoembolization with DC Bead™: results of 237 hepatocellular carcinoma (HCC) patients. Cardiovasc Intervent Radiol 34:774–785
Lencioni R, de Baere T, Burrel M et al (2012) Transcatheter treatment of hepatocellular carcinoma with doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol 35:980–985
Atassi B, Bangash AK, Lewandowski RJ et al (2008) Biliary sequelae following radioembolization with yttrium-90 microspheres. J Vasc Interv Radiol 19:691–697
Theysohn JM, Müller S, Schlaak JF et al (2013) Selective internal radiotherapy (SIRT) of hepatic tumors: how to deal with the cystic artery. Cardiovasc Intervent Radiol 36:1015–1022
Acknowledgments
Supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1220040).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chu, H.H., Kim, HC., Chung, J.W. et al. Repeated Intra-Arterial Therapy via the Cystic Artery for Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 37, 1283–1291 (2014). https://doi.org/10.1007/s00270-013-0795-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-013-0795-8