Abstract
Purpose
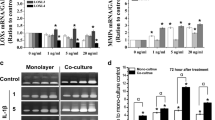
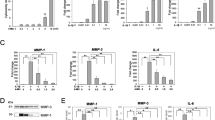
The adult human posterior cruciate ligament (PCL) has poor functional healing response. The synovial tissue, which surrounds the PCL ligament, might be the major regulator of the microenvironment in the joint cavity after PCL injury, thus affecting the healing process. Here we establish a novel co-culture system for PCL fibroblasts and synovial cells (SC) in vitro to explore the direct influence of paracrine on PCL cells by characterizing the different expressions of the lysyl oxidase family (LOXs) and matrix metalloproteinases (MMP-1, 2, 3), which respectively facilitate extracellular matrix (ECM) repair and degradation.
Methods
Total RNA was harvested, reverse transcribed and assessed by semi-quantitative PCR and real-time PCR for the expression of LOXs and MMP-1, 2, 3 messenger RNAs. MMP-2 activity was assayed from the collected culture media samples by using zymography.
Results
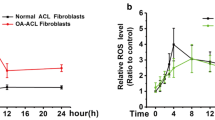
We found co-culture could promote gene expressions of the LOXs and MMP-1, 2, 3 in normal PCL fibroblasts. But in injured PCL, we found that matrix crosstalk induced an increase of the MMP-1, 2, 3 expressions and a down-regulation of the LOXs.
Conclusion
Based on these results, the crosstalk between PCL and SC strongly modified homeostatic balance of ECM and appeared to have a significant impact on PCL wound healing; decreased expression of cross-linking enzymes (LOXs) and increased expression of ECM-degrading proteinases (MMP-1, 2, 3) might be of great contribution to poor healing ability of PCL ligament.






Similar content being viewed by others
References
Mariani PP, Becker R, Rihn J et al (2003) Surgical treatment of posterior cruciate ligament and posterolateral corner injuries. An anatomical, biomechanical and clinical review. Knee 10:311–324
Tang Z, Yang L, Xue R et al (2009) Differential expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in anterior cruciate ligament and medial collateral ligament fibroblasts after a mechanical injury: Involvement of the p65 subunit of NF-κB. Wound Repair Regen 17:709–716
Ouyang HW, Goh JCH, Mo XM et al (2002) Characterization of anterior cruciate ligament cells and bone marrow stromal cells on various biodegradable polymericfilms. Mater Sci Eng C 20:63–69
Grassmayr MJ, Parker DA, Coolican MRJ et al (2008) Posterior cruciate ligament deficiency: Biomechanical and biological consequences and the outcomes of conservative treatment A systematic review. J Sci Med Sport 11:433–443
Keller PM, Shelbourne KD, McCarroll JR et al (1993) Nonoperatively treated isolated posterior cruciate ligament injuries. Am J Sports Med 21:132–136
Oiestad BE, Engebretsen L, Storheim K et al (2009) Knee osteoarthritis after anterior cruciate ligament injury a systematic review. Am J Sports Med 37:1434–1443
Woo SL, Chan SS, Yamaji T (1997) Biomechanics of knee ligament healing, repair and reconstruction. J Biomech 30:431–439
Girgis FG, Marshall JL, Monajem A (1975) The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clin Orthop Relat Res 106:216–231
Vandommelen BA, Fowler PJ (1989) Anatomy of the posterior cruciate ligament. A review. Am J Sports Med 17:24–29
Irie K, Uchiyama E, Iwaso H (2003) Intraarticular inflammatory cytokines in acute cruciate ligament injured knee. Knee 10:93–96
Tang Z, Yang L, Wang Y et al (2009) Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res 27:243–248
Oellers P, Schallenberg M, Stupp T et al (2009) A coculture assay to visualize and monitor interactions between migrating glioma cells and nerve fibers. Nat Protoc 4:923–927
Tandara AA, Mustoe TA (2011) MMP-and TIMP-secretion by human cutaneous keratinocytes and fibroblasts-impact of coculture and hydration. J Plast Reconstr Aesthet 64:108–116
Suzuki K, Saito J, Yanai R et al (2003) Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Prog Retin Eye Res 22:113–133
Wang HQ, Bai L, Shen BR, Yan ZQ et al (2007) Coculture with endothelial cells enhances vascular smooth muscle cell adhesion and spreading via activation of β1-integrin and phosphatidylinositol 3-kinase/Akt. Eur J Cell Biol 86:51–62
Zhou D, Lee HS, Villarreal F et al (2005) Differential MMP-2 activity of ligament cells under mechanical stretch injury: an in vitro study on human ACL and MCL fibroblasts. J Orthop Res 23:949–957
Xie J, Jiang JH, Zhang YJ et al (2011) Up-regulation expressions of lysyl oxidase family in anterior cruciate ligament and medial collateral ligament fibroblasts induced by transforming growth factor-beta. Int Orthop 36:207–213
Kim YH, Peyrol S, So CK et al (1999) Coexpression of the lysyl oxidase-like gene (LOXL) and the gene encoding type III procollagen in induced liver fibrosis. J Cell Biochem 72:181–188
Jourdan-Le Saux C, Tronecker H, Bogic L et al (1999) The LOXL2 gene encodes a new lysyl oxidase-like protein and is expressed at high levels in reproductive tissues. J Cell Biochem 274:12939–12944
Jourdan-Le Saux C, Tomsche A, Ujfalusi A et al (2001) Central nervous system, uterus, heart, and leukocyte expression of the LOXL3 gene, encoding a novel lysyl oxidase-like protein. Genomics 74:211–218
Asuncion L, Fogelgren B, Fong KSK et al (2001) A novel human lysyl oxidase-like gene (LOXL4) on chromosome 10q24 has an altered scavenger receptor cysteine rich domain. Matris Biol 20:487–491
Lucero HA, Kagan HM (2006) Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci 63:2304–2316
Gill SE, Parks WC (2008) Metalloproteinases and their inhibitors: Regulators of wound healing. Int J Biochem Cell Biol 40:1334–1347
Zhang J, Yang L, Tang Z et al (2009) Expression of MMPs and TIMPs family in human ACL and MCL fibroblasts. Connect Tissue Res 50:7–13
Hsieh AH, Tsai CMH, Ma QJ et al (2000) Time-dependent increase in type-III collagen gene expression in medial collateral ligament fibroblasts under cyclic strains. J Orthop Res 18:220–227
Steffensen B, Hakkinen L, Larjava H (2001) Proteolytic event of wound-healing coordinated interactions among matrix metalloproteinases (MMPs), integrins, and extracellular matrix molecules. Crit Rev Oral Biol Med 12:373–398
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839
Dandy DJ, Pusey RJ (1982) The long-term results of unrepaired tears of the posterior cruciate ligament. J Bone Joint Surg (Br) 64:92–94
Keller PM, Shelbourne KD, McCarroll JR et al (1993) Nonoperatively treated isolated posterior cruciate ligament injuries. Am J Sports Med 21:132–136
Andrish J, Holmes R (1979) Effects of synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat Res 279–283
Tang Z, Yang L, Wang Y et al (2009) Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res 27:243–248
Wang Y, Yang L, Zhang J et al (2009) Differential MMP-2 activity induced by mechanical compression and inflammatory factors in human synoviocytes. Mol Cell Biomech 7:105–114
Zhang Y, Huang W, Jiang J et al (2014) Influence of TNF-a and biomechanical stress on matrix metalloproteinases and lysyl oxidases expressions in human knee synovial fibroblasts. Knee Surg Sports Traumatol Arthrosc 22(9):1997–2006
Cameron ML, Fu FH, Paessler HH et al (1994) Synovial fluid cytokine concentrations as possible prognostic indicators in the ACL-deficient knee. Knee Surg Sports Traumatol Arthrosc 2:38–44
Kurpinski K, Chu J, Hashi C et al (2006) Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci USA 103:16095–16100
Lee AA, Delhaas T, McCulloch AD et al (1999) Differential responses of adult rat cardiac fibroblasts to in vitro biaxial strain patterns. J Mol Cell Cardiol 31:1833–1843
Lee AA, Delhaas T, Waldman LK et al (1996) An equibiaxial strain system for cultured cells. Am J Physiol Cell Physiol 271:C1400–C1408
Lee J, Harwood FL, Akeson WH et al (1998) Growth factor expression in healing rabbit medial collateral and anterior cruciate ligaments. Iowa Orthop J 18:19–25
Creemers LB, Jansen IDC, Docherty AJP et al (1998) Gelatinase A (MMP-2) and cysteine proteinases are essential for the degradation of collagen in soft connective tissue. Matrix Biol 17:35–46
Bramono DS, Richmond JC, Weitzel PP et al (2004) Matrix metalloproteinases and their clinical applications in orthopaedics. Clin Orthop Relat Res 428:272–285
Xie J, Wang C, Yin L et al (2013) IL-1β influences on lysyl oxidases and matrix metalloproteinases profile of injured anterior cruciate ligament and medial collateral ligament fibroblasts. Int Orthop 37(3):495–505
Wang Y, Tang Z, Xue R et al (2011) Combined effects of TNF-α, IL-1β, and HIF-1α on MMP-2 production in ACL fibroblasts under mechanical stretch: an in vitro study. J Orthop Res 29:1008–1014
Acknowledgments
This study was supported by the Innovation and Attracting Talents Program for College and University (“111” Project) (B06023), NSF Projects (10672195, 30870607), CSTC2008BB5192, Sharing Fund of Chongqing University’s large-scale equipment (2009063038) and by NIH AR45635 (USA).
Declaration of interests
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Xie, J., Jiang, J. et al. Differential expressions of the lysyl oxidase family and matrix metalloproteinases-1, 2, 3 in posterior cruciate ligament fibroblasts after being co-cultured with synovial cells. International Orthopaedics (SICOT) 39, 183–191 (2015). https://doi.org/10.1007/s00264-014-2573-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2573-x