Abstract
Coxsackievirus A10 (CVA10) recently has become one of the major pathogens of hand, foot, and mouth disease (HFMD) in children worldwide, but no cure or vaccine against CVA10 is available yet. Serological evaluation of herd immunity to CVA10 will promote the development of vaccine. The traditional neutralization assay based on inhibition of cytopathic effect (Nt-CPE) is a common method for measuring neutralizing antibody titer against CVA10, which is time-consuming and labor-intensive. In this study, an efficient neutralization test based on a monoclonal antibody (mAb) 3D1 against CVA10, called Elispot-based neutralization test (Nt-Elispot), was developed. In the Nt-Elispot, the mAb 3D1 labeled with horseradish peroxidase (HRP) was used to detect the CVA10-infected RD cells at a 1:4000 dilution and the optimal infectious dose of CVA10 was set at 105 TCID50/well when combined with a fixed incubation time of 14 h. Compared with the Nt-CPE, the Nt-Elispot method effectively shortened the detection period and presented a good correlativity with it. Using the Nt-Elispot, a total of 123 sera from healthy children were tested for neutralizing antibody against CVA10, demonstrating that the overall seroprevalence was 49.3% (54/123) and the geometric mean titer (GMT) had been calculated as 574.2. Furthermore, 2 anti-CVA10 neutralizing mAbs were obtained by screening via the Nt-Elispot. Overall, the established Nt-Elispot could be used as an efficient and high-throughput method for evaluating immunity to CVA10 and screening the neutralizing antibodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coxsackievirus A10 (CVA10) belongs to the Enterovirus genus of the Picornaviridae family (Itagaki et al. 1987), which has recently emerged as one of the main causative agents of hand, foot, and mouth disease (HFMD) in children. Sporadic occurrences and outbreaks of HFMD cases related by CVA10 have increased in Asia (Koh et al. 2016; Xing et al. 2014; Yang et al. 2015), Africa (Gopalkrishna et al. 2012), and Europe (Blomqvist et al. 2010; Bracho et al. 2011; Mirand et al. 2012) and resulted in a large burden of disease. In general, CVA10-associated HFMD is classically a mild disease, but some severe clinical manifestations such as onychomadesis (Bracho et al. 2011; Davia et al. 2011), convulsion (Chen et al. 2017), hyperCKemia (Okada et al. 2013), central nervous system disorders (Chen et al. 2017; Kumar et al. 2013), or even death were reported. Moreover, the co-infection and co-circulation of CVA10 with other enteroviruses can lead to more serious complications (Bracho et al. 2011). Vaccination has been considered the most effective strategy for HFMD control. Although some recent progress in developing vaccine (Lim et al. 2018; Shen et al. 2016) and therapy for CVA10 infection (Tan et al. 2018; Zhu et al. 2018b) have been reported, there is still no clinically available vaccine or cure accessible.
An efficient neutralization assay plays a major role in evaluating the efficacy of vaccines or antiviral reagents. The level of neutralizing antibody is regarded as a dominating indicator of protective immunity induced by vaccines or previous infection (Jin et al. 2016; Zhang et al. 2017; Zhu et al. 2018a). Traditionally, the standard neutralization test to detect neutralizing antibody levels against enteroviruses is the cytopathic effect (CPE)-based neutralization test (Nt-CPE) (WHO 1997). However, Nt-CPE has some shortcomings, such as long incubation periods and complex operations, and it is not suitable to apply to large sample tests. Therefore, an efficient and high-throughput neutralization assay needs to be developed.
The enzyme-linked immunospot (Elispot) assay has been verified as a highly sensitive, objective, and high-throughput immunological method (Ranieri et al. 2014). Each biomarker of interest produced in cell can sensitively be captured and detected by antibody, presenting as a spot via an enzyme-catalyzed color reaction. The number of antigen-specific cells is objectively determined by an Elispot analyzer, after automatically scanning and counting. In addition, this assay can be conducted in 96-well cell culture plates, which is greatly suitable for a high-throughput test. In recent years, several Elispot assays have been established for testing the level of neutralizing antibodies against Enterovirus 71 (EV71) (Mao et al. 2013), Coxsackievirus B3 (CVB3) (Yang et al. 2014), Coxsackievirus A16 (CVA16) (Hou et al. 2015), Echovirus (Echo25) (Li et al. 2016), EVD68 (Wan et al. 2017), Rotavirus (Li et al. 2014), and HSV-1 (Luo et al. 2016). However, this type of Elispot for CVA10 is not available yet. In this study, an efficient neutralization assay against CVA10, called Nt-Elispot, was successfully developed. A total of 123 sera from healthy children were tested for neutralizing antibody against CVA10 and 2 anti-CVA10 neutralizing mAbs were obtained by testing and screening using this assay.
Materials and methods
Cells, viruses, and serum samples
Rhabdomyosarcoma (RD) cells were obtained from the American Type Culture Collection (ATCC) and cultured in Minimal Essential Medium (MEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) plus 100 μg/mL streptomycin, 100 U/mL of penicillin, and 2 mM l-glutamine. The CVA10 strain CVA10-FJ-01 (GenBank accession no. KY012321) was isolated in Fujian Province of China, which was propagated in RD cells and was maintained in State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics (Xiamen University, China). The CVA10 titer was expressed as the 50% tissue culture infectious doses (TCID50), which was calculated by Reed-Muench method. The viruses were stored in aliquots at − 80 °C. An anti-CVA10 neutralizing antibody-positive human serum sample, an anti-CVA10 neutralizing antibody-negative human serum sample, and a total of 123 serum samples from healthy children aged between 8 months and 6 years in Xiang’an district of Xiamen City were provided by the Xiamen City Center for Disease Control and Prevention (XMCDC). The 123 serum samples were collected randomly in Xiang’an district in 2016. An anti-CVA10 neutralizing antibody-positive mice serum sample was collected from CVA10-immunized mice and an anti-CVA10 neutralizing antibody-negative mice serum sample were collected from non-immunized mice. All serum samples were stored at − 20 °C and inactivated at 56 °C for 30 min before testing.
Production of mAbs
BALB/c mice were obtained from Slac Laboratory Animal Co., Shanghai, China. Five 6-week-old female BALB/c mice were immunized subcutaneously with the CVA10-FJ-01 strain of CVA10, which was emulsified in an equal volume of Freund’s adjuvant (Sigma-Aldrich), and boosted twice at 2-week intervals. After the final boost, Sp2/0 myeloma cells were fused with spleen cells from immunized mice. The neutralization assay against CVA10 was applied for detecting all hybridoma supernatants. Antibodies were purified from the mouse ascitic fluid by protein A chromatography (GE Healthcare). All of the mAbs were conjugated to horseradish peroxidase (HRP) by the method that was reported by Nakane and Kawaoi (1974) and stored at − 20 °C.
Binding ELISA
The supernatant of CVA10 cultures (1:5 diluted) was adsorbed at 100 μL/well to 96-well plates at 4 °C overnight, then every well was blocked by 200 μL skim milk for 2 h at 37 °C after the wash with PBST (0.05% Tween-20 in PBS). Various concentrations of mAb 3D1 (the initial concentration of 1 mg/mL, diluted five-fold serially) were added and incubated at 37 °C for 1 h. After five times washing with PBST, GAM-HRP was added into each well as the secondary antibody at 1:5000 dilutions and incubated at 37 °C for 30 min. Following five times washing with PBST, the plant was treated with TMB chromogen substrate for color development and was stopped by 2 M H2SO4, absorbance was measured at A450/620. The average OD450 readings from triplicate wells at each dilution were read.
Immunofluorescence assay
RD cells were pre-seeded on round glass coverslips in 24-well tissue culture plates and then infected with CVA10. After 12 h, cells were fixed with 4% paraformaldehyde for 30 min in the dark. Then cells were permeabilized with 0.2% Triton X-100 diluted in PBS for 30 min at room temperature and blocked with 5% goat serum for 1 h at 37 °C. After washing with PBS, cells were incubated with mAb 3D1 (the initial concentration of 1 mg/mL at a dilution of 1:1000 in PBS) for 1 h at 37 °C. After three times washing with PBS, cells were stained with fluorescein isothiocyanate–conjugated goat anti-mouse antibody (GAM-FITC, at a dilution of 1:500 in PBS) for 30 min at 37 °C. After nucleus staining with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min at room temperature, cells were observed using a fluorescent microscope (BX51, Olympus).
Nt-CPE
RD cells were seeded at 5 × 103 cells per well into 96-well plates more than 6 h prior to the experiment. Serum samples were serially diluted two-fold with MEM and the first dilution was 16-fold. Each diluted serum was incubated in 1:1 volume ratio with CVA10 (100 TCID50) at 37 °C for 1 h and then added into pre-seeded cells. After incubation at 37 °C for 5–7 days, the CPE was observed by microscopy. All serum samples were tested in duplicate. The neutralizing titers were read as the highest dilution that completely inhibited CPE > 50% of the wells.
Nt-Elispot
RD cells were seeded at 2 × 104 cells per well into 96-well plates more than 6 h prior to the experiment. Serum samples or mAbs were serially diluted two-fold and incubated in 1:1 volume ratio with CVA10 (105 TCID50) at 37 °C for 1 h. The mixtures of samples and viruses were added into pre-seeded cells and then incubated at 37 °C for 14 h. All samples were tested in duplicate. For each plate, the cell control wells and the virus control wells should be set. After incubation, cells were detected by the Nt-Elispot assay as previously described (Yang et al. 2014). HRP-conjugated mAb 3D1 against CVA10 worked as the detection antibody at a 1:4000 dilution. After staining, the plates were scanned and the blue colored cells were counted by an ImmunoSpot Image analyzer 5.0 (Cellular Technology Ltd., USA). The inhibition rate of the sample was calculated using the following equation: P = [1 − (Ntest − Ncell control) / (Nvirus control − Ncell control)] × 100%. In this equation, P is the inhibition rate of the sample, and Ntest, Ncell control, and Nvirus control are the average number of spots in the test wells, cell control wells, and virus control wells respectively. The neutralization titer was defined as the highest dilution that completely inhibits > 50% of the spots.
Statistical analysis
The neutralizing titer, seroprevalence, and geometric mean titer (GMT) were calculated. All statistical analyses were performed using GraphPad Prism version 7.0.
Result
Characterizations of the monoclonal antibody 3D1
A panel of mAbs against CVA10 were acquired via the mice immunized with the supernatant of CVA10 cultures. A representative mAb 3D1, which exhibited high binding efficacy in ELISA (Fig. 1a), was selected as the detection antibody for the Nt-Elispot assay. The immunofluorescence assay showed that 3D1 had positive reactivity with CVA10-infected RD cells, but not with other enteroviruses species A such as EV71-, CVA16-, or CVA6-infected cells, and did not react with uninfected cells (Fig. 1b). Similarly, applied for the Nt-Elispot assay, the HRP-conjugated 3D1 (1 mg/mL) could only label the cells infected with CVA10 in blue, with the dilution of 1:4000 (Fig. 1b). These results indicated that mAb 3D1 was specific for CVA10.
Characterizations of the mAb 3D1. a Binding efficacy of 3D1 to CVA10 in ELISA. 96-Well plates were coated with CVA10 virus and diluted serially 3D1 were added. The amount of bound 3D1 was detected by HRP assay. The average OD450 readings from triplicate wells were displayed. b Specificity of the detection antibody 3D1. The results of immunofluorescence assay of CVA10-, EV71-, CVA16-, and CVA6-infected RD cells were shown in the upper line. The uninfected RD cells were used as negative control. The second antibody was GAM-FITC (green) and the nuclei were counterstained with DAPI (blue). The results of Nt-Elispot assay were displayed in the lower line. The CVA10-infected RD cells were labeled in blue with HRP-conjugated 3D1
Determination of the optimal infectious dose for the Nt-Elispot
The infection kinetic of CVA10, which was influenced by infectious dose and incubation time, should be determined for the Nt-Elispot. To shorten the testing time, RD cells were incubated with different doses of CVA10, ranging from 103 to 106 TCID50/well at 37 °C for 14 h. As shown in Fig. 2, the spots of the CVA10 group went up with the increasing of the infectious dose and peaked in the dose of 105 TCID50/well with about 1600 spots, and then began to decrease at higher doses of virus. The background was less than 100 spots in the dose of 105 TCID50/well in the inactivated CVA10 group and remained below 10 spots in the PBS group (Fig. 2). Thus, for the CVA10 Nt-Elispot, the optimal infectious dose was set at 105 TCID50/well when combined with a fixed incubation time of 14 h.
Determination of the optimal infectious dose for the Nt-Elispot. RD cells were incubated with different doses of CVA10, ranging from 103 to 106 TCID50/well. Inactivated-CVA10 (blue line) was heat-inactivated at 56 °C for 30 min. Both the inactivated-CVA10 and CVA10 were incubated at 37 °C for 14 h. Each of dilution well was performed in triplicate and followed by detection for the Nt-Elispot
Comparison of the Nt-CPE and the Nt-Elispot
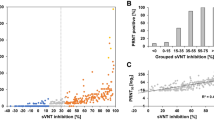
The traditional Nt-CPE is regarded as the standard method for detecting the neutralizing antibody level against enteroviruses and as a reference for evaluating other neutralization tests. In order to analyze the consistency between the Nt-CPE and Nt-Elispot, four samples of sera, a neutralizing antibody-positive human serum sample, a neutralizing antibody-negative human serum sample, a neutralizing antibody-positive mice serum sample, and a neutralizing antibody-negative mice serum sample were assayed. The neutralizing titers of these four samples were 2048, < 16, 8192, and < 16, respectively, as determined by the Nt-CPE. Then, these four serum samples were diluted serially in two-fold dilutions, tested by Nt-Elispot. The results showed that the neutralizing titers of both neutralizing antibody-positive sera were decreased with the increasing dilution at a rate of two times, and the measured neutralizing titers had a good correlation with the predicted neutralizing titers (Fig. 3a, c). In addition, as expected, the neutralizing titer of negative sera was < 16, tested by Nt-Elispot (Fig. 3b, d). Therefore, these data indicated that the results of Nt-Elispot were consistent with those of Nt-CPE, which indicated Nt-Elispot presented a good correlativity with Nt-CPE.
Comparison of the Nt-CPE and the Nt-Elispot. a A neutralizing antibody-positive human serum sample, b a neutralizing antibody-negative human serum sample, c a neutralizing antibody-positive mice serum sample, and d a neutralizing antibody-negative mice serum sample were assayed. The predicted neutralizing titers of these four samples were 2048, < 16, 8192, and < 16, respectively, as determined by the Nt-CPE. The serum samples were serially diluted in two-fold dilutions and analyzed by Nt-Elispot. The results of Nt-Elispot were consistent with those of Nt-CPE
Detection of serum neutralizing antibodies against CVA10 using Nt-Elispot
The infants and young children are susceptible to CVA10 infection. Using the Nt-Elispot, a total of 123 sera from healthy children aged between 8 months and 6 years were tested for neutralizing antibody against CVA10. A neutralizing titer of ≥ 16 was defined as a threshold value for positivity (Liu et al. 2011; Zhu et al. 2018a). As shown in Fig. 4a, the overall seroprevalence of CVA10 was 49.3% (54/123) and the geometric mean titer (GMT) has been calculated as 574.2. Furthermore, the seroprevalence was increased with age, ranging from approximately 20 to 70% (Fig. 4b), and the GMT was also increased with age and peaked in the age group of 3 to 4 years, with a neutralizing antibody titer of 301.9 (Fig. 4b). The results suggested that a high percentage of children had been exposed to CVA10 in Xiang’an district, especially those aged 3 to 4 years.
Detection of serum neutralizing antibodies against CVA10 using Nt-Elispot. a A total of 123 serum samples were collected from healthy population between 8 months and 6 years of age in Xiang’an district of Xiamen City. The neutralizing antibody titers against CVA10 were detected by Nt-Elispot. A neutralizing titer of ≥ 16 was defined as a threshold value for a positive score. b The Seroprevalence rate was illustrated via histogram. To show different levels of neutralizing antibody, the titers were divided into four ranges: ≥ 1:512 (high), 1:64–1:256 (moderate), 1:16–1:32 (low), and < 1:16 (no). The geometric mean titer (GMT) was calculated as the point plot noted
Screening of anti-CVA10 neutralizing antibody using Nt-Elispot
To gain the anti-CVA10 neutralizing antibodies, BALB/c mice were immunized with CVA10 and the splenocytes from the immunized mice were fused with sp2/0. Then, each 50 μL hybridoma supernatant was screened by Nt-Elispot against CVA10. After three rounds of screening and cloning, 2 anti-CVA10 neutralizing mAbs were obtained. As showed in Fig. 5a, mAb 7B4 and mAb 8D6 can effectively neutralize CVA10 in vitro, while the control anti-CVA6 mAb 1D5 (Xu et al. 2017) cannot. To determine the neutralizing titers of mAbs, 7B4 and 8D6 were diluted serially in two-fold dilutions (the initial concentration of 1 mg/mL), followed by Nt-Elispot. The results showed that the neutralizing titers of mAb 7B4 and 8D6 were 1024 and 512, respectively (Fig. 5b).
Screening of anti-CVA10 neutralizing antibody using Nt-Elispot. a The neutralizing efficacy of mAbs, 7B4, and 8D6. Both of them were detected by modified Nt-Elispot. 1D5, an antibody of CVA6, and PBS were used as controls. The CVA10-infected cells were labeled in blue with HRP-conjugated 3D1. b The neutralizing titers were calculated by Nt-Elispot as described in the material. Antibodies were diluted serially in two-fold dilutions (the initial concentration of 1 mg/mL)
Discussion
HFMD is a common infectious disease that mainly affects children under 5 years of age, leading to considerable morbidity and mortality worldwide. EV71 and CVA16 are previously the predominant pathogens of HFMD; however, a large number of HFMD cases associated with CVA10 infection were reported in recent years (Koh et al. 2016; Xing et al. 2014; Yang et al. 2015). Humoral immunity typically plays a central role in the defense of enterovirus infection (Chung et al. 2008). The evaluation of the neutralizing antibody levels against CVA10 in population is essential for understanding the status of herd immunity and facilitating the development of vaccines and antiviral reagents. The CPE-based neutralization assay remains the “gold standard” detection method for neutralizing antibody levels, but this traditional method is time-consuming and labor-intensive, which is not applicable to test a variety of samples. In this study, we developed a neutralization assay (Nt-Elispot) based on a specific mAb 3D1 against CVA10. Compared to the Nt-CPE, the incubation time of this experiment is shortened from more than 3–5 days to 14 h. The Nt-Elispot quantifies the CVA10-infected cells based on the expression of viral protein, which can be bound by HRP-labeled mAb 3D1. After staining, an Elispot analyzer can automatically scan and count the stained plaques without detection under a microscope. Therefore, the results are more stable and objective. Moreover, this method can be performed in 96-well cell culture plates, which is favorable for high-throughput detection. Previous studies demonstrated that there was a negative linear correlation between the infectious dose and the incubation time (Yang et al. 2014). For convenience and decrease in the testing time, the optimal CVA10 infectious dose and the incubation time were set at 105 TCID50/well and 14 h, respectively.
Xiamen is a coastal tourist city in Fujian Province, which is divided into six districts, with approximately 27,000 cases of HFMD reported from 2008 to 2015 (He et al. 2017). Moreover, Chen et al. found that the proportion of CVA10 infections in severe HFMD cases in Xiamen City increased sharply from 0% in 2011, 2012, and 2014 to 38.7% in 2015 (Chen et al. 2017). Among six districts in Xiamen, the annual morbidity of overall HFMD cases in Xiang’an district ranked second, with a rate of 1400/1,000,000 (He et al. 2017). In our previous study, we conducted a serological survey of neutralizing antibodies to eight major enteroviruses among healthy people aged 5 months to 83 years, which found that the overall seroprevalence of CVA10 was 42.7% in Xiamen City and the seroprevalence in Xiang’an district was the highest with a rate of 57.3% (Zhu et al. 2018a). These results demonstrated that CVA10 may be one of the emerging agents of HFMD in Xiamen City, especially in Xiang’an district. The infants and young children are more susceptible to HFMD. To further characterize the seroepidemiology of CVA10 among children, in this study, we tested the neutralizing antibodies against CVA10 among 123 healthy children aged 8 months and 6 years in Xiang’an district by Nt-Elispot. The result showed that the overall seroprevalence of CVA10 among children was 49.3%, which further indicated that CVA10 was prevalent in the region.
In summary, the established CVA10 Nt-Elispot can be a sensitive, easy, and high-throughput method for anti-CVA10 neutralizing antibodies detection and served as a useful tool for CVA10 vaccine evaluation and serology surveillance. Furthermore, positive hybridoma supernatant against CVA10 could be objectively screened by Nt-Elispot.
References
Blomqvist S, Klemola P, Kaijalainen S, Paananen A, Simonen ML, Vuorinen T, Roivainen M (2010) Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol 48(1):49–54. https://doi.org/10.1016/j.jcv.2010.02.002
Bracho MA, Gonzalez-Candelas F, Valero A, Cordoba J, Salazar A (2011) Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg Infect Dis 17(12):2223–2231. https://doi.org/10.3201/eid1712.110395
Chen M, He S, Yan Q, Xu X, Wu W, Ge S, Zhang S, Chen M, Xia N (2017) Severe hand, foot and mouth disease associated with Coxsackievirus A10 infections in Xiamen, China in 2015. J Clin Virol 93:20–24. https://doi.org/10.1016/j.jcv.2017.05.011
Chung YC, Ho MS, Wu JC, Chen WJ, Huang JH, Chou ST, Hu YC (2008) Immunization with virus-like particles of enterovirus 71 elicits potent immune responses and protects mice against lethal challenge. Vaccine 26(15):1855–1862. https://doi.org/10.1016/j.vaccine.2008.01.058
Davia JL, Bel PH, Ninet VZ, Bracho MA, Gonzalez-Candelas F, Salazar A, Gobernado M, Bosch IF (2011) Onychomadesis outbreak in Valencia, Spain associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol 28(1):1–5. https://doi.org/10.1111/j.1525-1470.2010.01161.x
Gopalkrishna V, Patil PR, Patil GP, Chitambar SD (2012) Circulation of multiple enterovirus serotypes causing hand, foot and mouth disease in India. J Med Microbiol 61(Pt 3):420–425. https://doi.org/10.1099/jmm.0.036400-0
He S, Chen M, Xu X, Yan Q, Niu J, Wu W, Su X, Ge S, Zhang S, Xia N (2017) Epidemics and aetiology of hand, foot and mouth disease in Xiamen, China, from 2008 to 2015. Epidemiol Infect 145(9):1865–1874. https://doi.org/10.1017/S0950268817000309
Hou W, Yang L, He D, Zheng J, Xu L, Liu J, Liu Y, Zhao H, Ye X, Cheng T, Xia N (2015) Development of a coxsackievirus A16 neutralization test based on the enzyme-linked immunospot assay. J Virol Methods 215-216:56–60. https://doi.org/10.1016/j.jviromet.2015.02.010
Itagaki A, Kamahora T, Kurimura T (1987) Isolation and characterization of a cold-sensitive strain of coxsackievirus A10. J Gen Virol 68(Pt 4):1191–1194. https://doi.org/10.1099/0022-1317-68-4-1191
Jin P, Li J, Zhang X, Meng F, Zhou Y, Yao X, Gan Z, Zhu F (2016) Validation and evaluation of serological correlates of protection for inactivated enterovirus 71 vaccine in children aged 6-35 months. Hum Vaccin Immunother 12(4):916–921. https://doi.org/10.1080/21645515.2015.1118595
Koh WM, Bogich T, Siegel K, Jin J, Chong EY, Tan CY, Chen MI, Horby P, Cook AR (2016) The epidemiology of hand, foot and mouth disease in Asia: a systematic review and analysis. Pediatr Infect Dis J 35(10):e285–e300. https://doi.org/10.1097/INF.0000000000001242
Kumar A, Shukla D, Kumar R, Idris MZ, Jauhari P, Srivastava S, Dhole TN (2013) Molecular identification of enteroviruses associated with aseptic meningitis in children from India. Arch Virol 158(1):211–215. https://doi.org/10.1007/s00705-012-1476-7
Li T, Lin H, Yu L, Xue M, Ge S, Zhao Q, Zhang J, Xia N (2014) Development of an enzyme-linked immunospot assay for determination of rotavirus infectivity. J Virol Methods 209:7–14. https://doi.org/10.1016/j.jviromet.2014.08.012
Li S, Yang L, Hou W, Zhao H, Wan J, Chen M, HE S, Cheng T, Xia N (2016) Development and application of a novel neutralization test for echovirus 25. Lett Microbiol 1009–0002
Lim H, In HJ, Lee JA, Sik Yoo J, Lee SW, Chung GT, Choi YK, Chung JK, Cho SJ, Lee JW (2018) The immunogenicity and protection effect of an inactivated coxsackievirus A6, A10, and A16 vaccine against hand, foot, and mouth disease. Vaccine 36(24):3445–3452. https://doi.org/10.1016/j.vaccine.2018.05.005
Liu CC, Guo MS, Lin FH, Hsiao KN, Chang KH, Chou AH, Wang YC, Chen YC, Yang CS, Chong PC (2011) Purification and characterization of enterovirus 71 viral particles produced from vero cells grown in a serum-free microcarrier bioreactor system. PLoS One 6(5):e20005. https://doi.org/10.1371/journal.pone.0020005
Luo Y, Xiong D, Li H, Qiu S, Lin C, Chen Q, Huang C, Yuan Q, Zhang J, Xia N (2016) Development of an HSV-1 neutralization test with a glycoprotein D specific antibody for measurement of neutralizing antibody titer in human sera. Virol J 13:44. https://doi.org/10.1186/s12985-016-0508-4
Mao Q, Cheng T, Zhu F, Li J, Wang Y, Li Y, Gao F, Yang L, Yao X, Shao J, Xia N, Liang Z, Wang J (2013) The cross-neutralizing activity of enterovirus 71 subgenotype c4 vaccines in healthy chinese infants and children. PLoS One 8(11):e79599. https://doi.org/10.1371/journal.pone.0079599
Mirand A, Henquell C, Archimbaud C, Ughetto S, Antona D, Bailly JL, Peigue-Lafeuille H (2012) Outbreak of hand, foot and mouth disease/herpangina associated with coxsackievirus A6 and A10 infections in 2010, France: a large citywide, prospective observational study. Clin Microbiol Infect 18(5):E110–E118. https://doi.org/10.1111/j.1469-0691.2012.03789.x
Nakane PK, Kawaoi A (1974) Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem 22(12):1084–1091. https://doi.org/10.1177/22.12.1084
Okada H, Wada M, Sato H, Yamaguchi Y, Tanji H, Kurokawa K, Kawanami T, Takahashi T, Kato T (2013) Neuromyelitis optica preceded by hyperCKemia and a possible association with coxsackie virus group A10 infection. Intern Med 52(23):2665–2668
Ranieri E, Popescu I, Gigante M (2014) CTL ELISPOT assay. Methods Mol Biol 1186:75–86. https://doi.org/10.1007/978-1-4939-1158-5_6
Shen C, Liu Q, Zhou Y, Ku Z, Wang L, Lan K, Ye X, Huang Z (2016) Inactivated coxsackievirus A10 experimental vaccines protect mice against lethal viral challenge. Vaccine 34(41):5005–5012. https://doi.org/10.1016/j.vaccine.2016.08.033
Tan YW, Yam WK, Sun J, Chu JJH (2018) An evaluation of Chloroquine as a broad-acting antiviral against Hand, Foot and Mouth Disease. Antivir Res 149:143–149. https://doi.org/10.1016/j.antiviral.2017.11.017
Wan J, Wang W, Hou W, Li S, Zhao H, Zheng Q, He S, Cheng T, Xia N (2017) Development and application of a novel neutralization test for enterovirus D68. Chin J Virol
WHO (1997) Manual for the virological investigation of polio Global programme for vaccines and immunization, expanded programme on immunization. World Health Organization, Geneva
Xing W, Liao Q, Viboud C, Zhang J, Sun J, Wu JT, Chang Z, Liu F, Fang VJ, Zheng Y, Cowling BJ, Varma JK, Farrar JJ, Leung GM, Yu H (2014) Hand, foot, and mouth disease in China, 2008-12: an epidemiological study. Lancet Infect Dis 14(4):308–318. https://doi.org/10.1016/S1473-3099(13)70342-6
Xu L, Zheng Q, Li S, He M, Wu Y, Li Y, Zhu R, Yu H, Hong Q, Jiang J, Li Z, Li S, Zhao H, Yang L, Hou W, Wang W, Ye X, Zhang J, Baker TS, Cheng T, Zhou ZH, Yan X, Xia N (2017) Atomic structures of Coxsackievirus A6 and its complex with a neutralizing antibody. Nat Commun 8(1):505. https://doi.org/10.1038/s41467-017-00477-9
Yang L, He D, Tang M, Li Z, Liu C, Xu L, Chen Y, Du H, Zhao Q, Zhang J, Cheng T, Xia N (2014) Development of an enzyme-linked immunosorbent spot assay to measure serum-neutralizing antibodies against coxsackievirus B3. Clin Vaccine Immunol 21(3):312–320. https://doi.org/10.1128/CVI.00359-13
Yang Q, Ding J, Cao J, Huang Q, Hong C, Yang B (2015) Epidemiological and etiological characteristics of hand, foot, and mouth disease in Wuhan, China from 2012 to 2013: outbreaks of coxsackieviruses A10. J Med Virol 87(6):954–960. https://doi.org/10.1002/jmv.24151
Zhang D, Chen Y, Chen X, He Z, Zhu X, Hao Y (2017) Enterovirus 71 neutralizing antibodies seroepidemiological research among children in Guangzhou, China between 2014 and 2015: a cross-sectional study. Int J Environ Res Public Health 14(3). https://doi.org/10.3390/ijerph14030319
Zhu R, Cheng T, Yin Z, Liu D, Xu L, Li Y, Wang W, Liu J, Que Y, Ye X, Tang Q, Zhao Q, Ge S, He S, Xia N (2018a) Serological survey of neutralizing antibodies to eight major enteroviruses among healthy population. Emerg Microbes Infect 7(1):2. https://doi.org/10.1038/s41426-017-0003-z
Zhu R, Xu L, Zheng Q, Cui Y, Li S, He M, Yin Z, Liu D, Li S, Li Z, Chen Z, Yu H, Que Y, Liu C, Kong Z, Zhang J, Baker TS, Yan X, Hong Zhou Z, Cheng T, Xia N (2018b) Discovery and structural characterization of a therapeutic antibody against coxsackievirus A10. Sci Adv 4(9):eaat7459. https://doi.org/10.1126/sciadv.aat7459
Acknowledgments
We thank the editors at NPG Language Editing who provided editing assistance to the authors during the preparation of this manuscript.
Funding
This work was supported by a grant from the National Science and Technology Major Project of Infectious Diseases (No.2017ZX10304402), the National Natural Science Foundation of China (Nos. 81801646, 31670933, and 81701999), and the National Science and Technology Major Projects for Major New Drugs Innovation and Development (No. 2018ZX09711003-005-003). The sponsors had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animal experiments were approved by Xiamen University Laboratory Animal Center (XMULAC) and conducted in accordance with animal ethics guidelines and approved protocols. The Animal Ethics Committee approval number was XMULAC20160049. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committees of the Xiamen City Center for Disease Control and Prevention and the Xiamen University.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, D., Xu, L., Zhu, R. et al. Development of an efficient neutralization assay for Coxsackievirus A10. Appl Microbiol Biotechnol 103, 1931–1938 (2019). https://doi.org/10.1007/s00253-018-09598-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-09598-7