Abstract
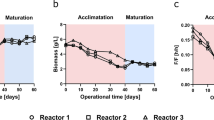
Physiological heterogeneity constitutes a critical parameter in biotechnological systems since both metabolite yield and productivity are often hampered by the presence of undesired physiological cell subpopulations. In the present study, the physiological status and functionality of Pseudomonas taetrolens cells were monitored by multiparameter flow cytometry during fermentative lactobionic acid production at the shake-flask and bioreactor scale. In shake-flask fermentation, the onset of the lactobionic acid production phase was accompanied by a progressive loss of cellular metabolic activity, membrane polarization, and membrane integrity concomitantly to acidification. In fact, population dynamics has shown the prevalence of damaged and dead subpopulations when submitted to a pH < 4 from 16 h onwards. Furthermore, fluorescence-activated cell sorting revealed that these sublethally injured cells were nonculturable. In contrast, P. taetrolens cells exhibited a robust physiological status during bioreactor cultivations performed with a pH-shifted strategy at 6.5, remaining predominantly healthy and metabolically active (>96 %) as well as maintaining bioconversion efficiency throughout the course of the fermentation. Additionally, an assessment of the seed culture’s physiological robustness was carried out in order to determine the best seed culture age. Results showed that bioreactor culture performance, growth, and lactobionic acid production efficiency were strongly dependent on the physiological heterogeneity displayed by the seed culture. This study provides the most suitable criteria for optimizing lactobionic acid production efficiency through a novel flow cytometric-based approach based on the physiological status of P. taetrolens. It also constitutes a valuable, broad-ranging methodology for the enhancement of microbial bioprocesses involved in the production of secondary metabolites.








Similar content being viewed by others
References
Alonso S, Rendueles M, Díaz M (2011) Efficient lactobionic acid production from whey by Pseudomonas taetrolens under pH-shift conditions. Bioresour Technol 102(20):9730–9736
Alonso S, Rendueles M, Díaz M (2012) Role of dissolved oxygen availability on lactobionic acid production from whey by Pseudomonas taetrolens. Bioresour Technol 109:140–147
Amanullah A, Hewitt CJ, Nienow AW, Lee C, Chartrain M, Buckland BC, Drew SW, Woodley JM (2003) Measurement of strain-dependent toxicity in the indene bioconversion using multiparameter flow cytometry. Biotechnol Bioeng 81(4):405–420
Amor BK, Breeuwer P, Verbaarschot P, Rombouts FM, Akkermans AD, De Vos WM, Abee T (2002) Multiparametric flow cytometry and cell sorting for the assessment of viable, injured, and dead Bifidobacterium cells during bile salt stress. Appl Environ Microb 68(11):5209–5216
Avery SV (2006) Microbial cell individuality and the underlying sources of heterogeneity. Nat Rev Microbiol 4(8):577–587
Bandyopadhyay B, Humphrey AE, Taguchi H. Introduction by Rao G (2009) Dynamic measurement of the volumetric oxygen transfer coefficient in fermentation systems. Biotechnol Bioeng 104(5):841–853
Bogosian G, Bourneuf EV (2001) A matter of bacterial life and death. EMBO Rep 2(9):770–774
David F, Berger A, Hänsch R, Rohde M, Franco-Lara E (2011) Single cell analysis applied to antibody fragment production with Bacillus megaterium: development of advanced physiology and bioprocess state estimation tools. Microb Cell Fact 10:23
Díaz M, Herrero M, García LA, Quirós C (2010) Application of flow cytometry to industrial microbial bioprocesses. Biochemical Eng J 48(3):385–407
Enfors SO, Jahic M, Rozkov A, Xu B, Hecker M, Jürgen B, Krüger E, Schweder T, Hamer G, O’Beirne D, Noisommit-Rizzi N, Reuss M, Boone L, Hewitt C, McFarlane C, Nienow A, Kovacs T, Trägårdh C, Fuchs L, Revstedt J, Friberg PC, Hjertager B, Blomstein H, Hjort S, Hoeks F, Lin HY, Neubauer P, van der Lans R, Luyben K, Vrabel P, Manelius Å (2001) Physiological responses to mixing in large scale bioreactors. J Biotechnol 85(2):175–185
Green BA, Yu RJ, Van Scott EJ (2009) Clinical and cosmeceutical uses of hydroxyacids. Clin Dermatol 27(5):495–501
Hammes F, Berney M, Egli T (2011) Cultivation-independent assessment of bacterial viability. Adv Biochem Eng Biot 124:123–150
Herrero M, Quirós C, García LA, Díaz M (2006) Use of flow cytometry to follow the physiological states of microorganisms in cider fermentation processes. Appl Environ Microbiol 72(10):6725–6733
Hewitt CJ, Nebe-von-Caron G (2001) An industrial application of multiparameter flow cytometry: assessment of cell physiological state and its application to the study of microbial fermentations. Cytometry 44(3):179–187
Hewitt CJ, Nebe-von-Caron G (2004) The application of multi-parameter flow cytometry to monitor individual microbial cell physiological state. Adv Biochem Eng Biot 89:197–223
Hewitt CJ, Nebe-von-Caron G, Nienow AW, McFarlane CM (1999) The use of multi-parameter flow cytometry to compare the physiological response of Escherichia coli W3110 to glucose limitation during batch, fed-batch and continuous culture cultivations. J Biotechnol 75(2–3):251–264
Kottmeier K, Weber J, Müller C, Bley T, Büchs J (2009) Asymmetric division of Hansenula polymorpha reflected by a drop of light scatter intensity measured in batch microtiter plate cultivations at phosphate limitation. Biotechnol Bioeng 104(3):554–561
Lin WJ, Chen TD, Liu CW, Chen JL, Chang FH (2011) Synthesis of lactobionic acid-grafted-pegylated-chitosan with enhanced HepG2 cells transfection. Carbohydr Polym 83(2):898–904
Lopes da Silva T, Reis A, Kent CA, Kosseva M, Roseiro JC, Hewitt CJ (2005) Stress-induced physiological responses to starvation periods as well as glucose and lactose pulses in Bacillus licheniformis CCMI 1034 continuous aerobic fermentation processes as measured by multi-parameter flow cytometry. Biochemical Eng J 24(1):31–41
Lopes da Silva T, Piekova L, Mileu J, Roseiro JC (2009) A comparative study using the dual staining flow cytometric protocol applied to Lactobacillus rhamnosus and Bacillus licheniformis batch cultures. Enzyme Microb Tech 45(2):134–138
Lüders S, David F, Steinwand M, Jordan E, Hust M, Dübel S, Franco-Lara E (2011) Influence of the hydromechanical stress and temperature on growth and antibody fragment production in Bacillus megaterium. Appl Microbiol Biot 91(1):81–90
Müller S, Vogt C, Laube M, Harms H, Kliensteuber S (2009) Community dynamics within a bacterial consortium during growth on toluene under sulfate-reducing conditions. FEMS Microbiol Rev 70(3):586–596
Müller S, Harms H, Bley T (2010) Origin and analysis of microbial population heterogeneity in bioprocesses. Curr Opin Biotech 21(1):100–113
Navarro JM, Tormo A, Martínez-García E (2010) Stationary phase in gram-negative bacteria. FEMS Microbiol Rev 34(4):476–495
Nicolau SA, Gaida SM, Papoutsakis ET (2010) A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: from biofuels and chemicals, to biocatalysis and bioremediation. Metab Eng 12(4):307–331
Nielsen TH, Sjøholm OR, Sørensen J (2009) Multiple physiological states of a Pseudomonas fluorescens DR54 biocontrol inoculant monitored by a new flow cytometry protocol. FEMS Microbiol Ecol 67(3):479–490
Nyström T (2005) Bacterial senescence, programmed death, and premeditated sterility. ASM News 71:363–369
Papadimitriou K, Pratsinis H, Neve-von-Caron G, Kletsas D, Tsakalidou E (2006) Rapid assessment of the physiological status of Streptococcus macedonicus by flow cytometry and fluorescence probes. Int J Food Microbiol 111(3):197–205
Papadimitriou K, Pratsinis H, Neve-von-Caron G, Kletsas D, Tsakalidou E (2007) Acid tolerance of Streptococcus macedonicus as assessed by flow cytometry and single-cell sorting. Appl Environ Microb 73(2):465–476
Want A, Hancocks H, Thomas CR, Stocks SM, Nebe-von-Caron G, Hewitt CJ (2011) Multi-parameter flow cytometry and cell sorting reveal extensive physiological heterogeneity in Bacillus cereus batch cultures. Biotechnol Lett 33(7):1395–1405
Zhang Y, Zhu Y, Zhu Y, Li Y (2009) The importance of engineering physiological functionality into microbes. Trends Biotechnol 27(12):664–672
Zhang J, Li C, Xue ZY, Cheng HW, Huang FW, Zhuo RX, Zhang XZ (2011) Fabrication of lactobionic-loaded chitosan microcapsules as potential drug carriers targeting the liver. Acta Biomater 7(4):1665–1673
Zhu J, Thakker C, San KY, Bennet G (2011) Effect of culture operating conditions on succinate production in a multiphase fed-batch bioreactor using an engineered Escherichia coli strain. Appl Microbiol Biot 92(3):499–508
Acknowledgments
The authors are grateful for the financial support from the Spanish Ministry of Science and Innovation through project MEC-CTQ2010-14918. The technical assistance of Ana Salas, Magdalena Choda (Flow Cytometry Area, Scientific-Technical Services, University of Oviedo), and Marta Alonso (Process Image Area, Scientific-Technical Services, University of Oviedo) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alonso, S., Rendueles, M. & Díaz, M. Physiological heterogeneity of Pseudomonas taetrolens during lactobionic acid production. Appl Microbiol Biotechnol 96, 1465–1477 (2012). https://doi.org/10.1007/s00253-012-4254-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4254-2