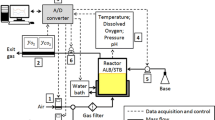
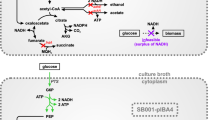
Abstract
A metabolically engineered Escherichia coli strain SBS550MG (pHL413) was used in this study to investigate the impact of various culture operating conditions for improving the specific succinate production rate for better final titer while maintaining the theoretical succinate yield on glucose in multiphase fed-batch cultures. Previously, we reported that changes in the level of aeration during the cell growth phase significantly modified gene expression profiles and metabolic fluxes in this system (Martinez et al. 2010). Based on these observations, the examination of culture conditions was mainly focused on the aerobic growth phase. It was found that 2–5 h of low dissolved oxygen culture during the aerobic phase improves cell productivity, but pH control during the aerobic phase was not favorable for the system. Cell viability has been identified as a major limiting factor for succinate production. Supplementing LB medium and betaine, an anti-osmotic stress reagent, did not improve cell activity. A higher succinate titer (537.8 mM) using the current metabolic engineering E. coli strain was achieved, which can potentially be improved further by increasing cell viability.








Similar content being viewed by others
References
Andersson C, Helmerius J, Hodge D, Berglund KA, Rova U (2009) Inhibition of succinic acid production in metabolically engineered Escherichia coli by neutralizing agent, organic acids, and osmolarity. Biotechnol Prog 25(1):116–123
Baneyx F (1999) Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol 10(5):411–421
Berrios-Rivera SJ, Bennett GN, San KY (2002) The effect of increasing NADH availability on the redistribution of metabolic fluxes in Escherichia coli chemostat cultures. Metab Eng 4(3):230–237
Böck A, Curtiss R III, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM, Squires CL, Ussery D (2008) EcoSal-Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington DC
Cox SJ, Shalel Levanon S, Sanchez A, Lin H, Peercy B, Bennett GN, San KY (2006) Development of a metabolic network design and optimization framework incorporating implementation constraints: a succinate production case study. Metab Eng 8:46–57
Cozzone AJ (1998) Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annu Rev Microbiol 52:127–164
Haacke A, Fendrich G, Ramage P, Geiser M (2009) Chaperone over-expression in Escherichia coli: apparent increased yields of soluble recombinant protein kinases are due mainly to soluble aggregates. Protein Expr Purif 64(2):185–193
Haussinger D, Lang F (1991) Cell volume in the regulation of hepatic function: a mechanism for metabolic control. Biochimica Et Biophysica Acta 1071(4):331–50
Hong SH, Lee SY (2002) Importance of redox balance on the production of succinic acid by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 58(3):286–290
Jantama K, Haupt MJ, Svoronos SA, Zhang X, Moore JC, Shanmugam KT, Ingram LO (2008) Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of Escherichia coli C that produce succinate and malate. Biotechnol Bioeng 99(5):1140–1153
Larsen PI, Sydnes LK, Landfald B, Strom AR (1987) Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch Microbiol 147:1–7
Lee PC, Lee WG, Lee SY, Chang HN (2001) Succinic acid production with reduced by-product formation in the fermentation of Anaerobiospirillum succiniciproducens using glycerol as a carbon source. Biotechnol Bioeng 72(1):41–48
Lee PC, Lee SY, Hong SH, Chang HN, Park SC (2003) Biological conversion of wood hydrolysate to succinic acid by Anaerobiospirillum succiniciproducens. Biotechnol Lett 25(2):111–114
Lee SJ, Song H, Lee SY (2006) Genome-based metabolic engineering of Mannheimia succiniciproducens for succinic acid production. Appl Environ Microbiol 72(3):1939–1948
Lynch AS, Lin EC (1996) Transcriptional contorl mediated by the ArcA two-component response regulator protein of Eschericha coli: characterization of DNA binding at target promoters. J Bacteriol 178:6238–6249
Martinez I, Bennett GN, San KY (2010) Metabolic impact of the level of aeration during cell growth on anaerobic succinate production by an engineered Escherichia coli strain. Metab Eng 12:499–509
McKinlay JB, Zeikus JG, Vieille C (2005) Insights into Actinobacillus succinogenes fermentative metabolism in a chemically defined growth medium. Appl Environ Microbiol 71(11):6651–6656
Oh IJ, Lee HW, Park CH, Lee SY, Lee J (2008) Succinic acid production by continuous fermentation process using Mannheimia succiniciproducens LPK7. J Microbiol Biotechnol 18(5):908–912
Otero JM, Olsson L, Nielsen J (2007) Metabolic engineering of Saccharomyces cerevisiae microbial cell factories for succinic acid production. J Biotechnol 131(2):S205–S205
Portle S, Causey TB, Wolf K, Bennett GN, San KY, Mantzaris N (2007) Cell population heterogeneity in expression of a gene-switching network with fluorescent markers of different half-lives. J Biotechnol 128(2):362–375
Robinson AC, Burgess BK, Dean DR (1986) Activity, reconstitution, and accumulation of nitrogenase components in Azotobacter vinelandii mutant strains containing defined deletions within the nitrogenase structural gene cluster. J Bacteriol 166(1):180–186
Sanchez AM, Bennett GN, San KY (2005) Novel pathway engineering design of the anaerobic central metabolic pathway in Escherichia coli to increase succinate yield and productivity. Metab Eng 7(3):229–239
Sanchez AM, Bennett GN, San KY (2006) Batch culture characterization and metabolic flux analysis of succinate-producing Escherichia coli strains. Metab Eng 8:209–226
Underwood SA, Buszko MT, Shanmugam KT, Ingram LO (2004) Lack of protective osmolytes limits final cell density and volumetric productivity of ethanologenic Escherichia coli KO11 during xylose fermentation. Appl Environ Microbiol 70:2734–2740
Vemuri GN, Eiteman MA, Altman E (2002) Succinate production in dual-phase Escherichia coli fermentations depends on the time of transition from aerobic to anaerobic conditions. J Ind Microbiol Biotechnol 28(6):325–332
Walsh K, Koshland DE (1985) Branch point control by the phosphorylation state of isocitrate dehydrogenase. A quantitative examination of fluxes during a regulatory transition. J Biol Chem 260(14):8430–8437
Werpy T, Petersen G (2004) Top value-added chemicals from biomass—volume I. US Department of Energy, Washington, DC
Yang YT, Bennett GN, San KY (1999) Effect of inactivation of nuo and ackA-pta on redistribution of metabolic fluxes in Escherichia coli. Biotechnol Bioeng 65(3):291–297
Zhou S, Grabar TB, Shanmugam KT, Ingram LO (2006) Betaine tripled the volumetric productivity of D (–)-lactate by Escherichia coli strain SZ132 in mineral salts medium. Biotechnol Lett 28:671–676
Acknowledgment
The authors appreciate Mary Harrison for her creative and reliable support on the lab management and technical advice. We also thank Irene Martinez for her constructive discussions and suggestions on the experiments. This work was supported by funding from ROQUETTE FRERES, France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, J., Thakker, C., San, KY. et al. Effect of culture operating conditions on succinate production in a multiphase fed-batch bioreactor using an engineered Escherichia coli strain. Appl Microbiol Biotechnol 92, 499–508 (2011). https://doi.org/10.1007/s00253-011-3314-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3314-3