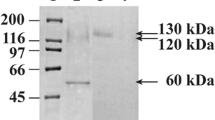
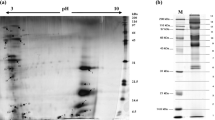
Abstract
Saccharomyces cerevisiae Sta1 glucoamylase and Saccharomycopsis fibuligera Bgl1 β-glucosidase, two relevant enzymes from a biotechnological point of view, are proteins with multidomain structure. Starting with homology-based structural models of Sta1 and Bgl1, we have constructed a series of hybrid enzymes by interchanging domains of the two proteins. The first purpose of these constructs was to check available hypotheses about the uncertain biological functions of two domains: the serine/threonine-rich domain (STRD) of Sta1 and a β-sandwich domain present in Bgl1 that we have designated fibronectin-like domain (FLD). While, according to the initial hypothesis, proteins carrying the FLD tend to adhere to the cell wall, our results argued against the idea of an involvement of the STRD in protein secretion that stemmed from the presence of similar domains in different proteins secreted by yeast. The second objective of this work was to increase the enzymatic repertoire by generating enzymes with new structural and functional properties.






Similar content being viewed by others
References
Adam AC, Latorre-Garcia L, Polaina J (2004) Structural analysis of glucoamylase encoded by the STA1 gene of Saccharomyces cerevisiae (var. diastaticus). Yeast 21:379–388
Arrizubieta MJ, Polaina J (2000) Increased thermal resistance and modification of the catalytic properties of a β-glucosidase by random mutagenesis and in vitro recombination. J Biol Chem 275:28843–28848
Boraston AB, Bolam DN, Gilbert HJ, Davies GJ (2004) Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem J 382:769–781
Bryson K, McGuffin LJ, Marsden RL, Ward JJ, Sodhi JS, Jones DT (2005) Protein structure prediction servers at University College London. Nucleic Acids Res 33:W36–W38
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The carbohydrate-active enzymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238
Cesareni G, Murray AH (1987) Plasmid vector carrying the replication origin of filamentous single-stranded phages. In: Setlow JK (ed) Genetic engineering. Plenum, New York, pp 135–154
Chothia C, Gough J (2009) Genomic and structural aspects of protein evolution. Biochem J 419:15–28
Den HR, Rose SH, Lynd LR, van Zyl WH (2007) Hydrolysis and fermentation of amorphous cellulose by recombinant Saccharomyces cerevisiae. Metab Eng 9:87–94
Fischbach MA, Lai JR, Roche ED, Walsh CT, Liu DR (2007) Directed evolution can rapidly improve the activity of chimeric assembly-line enzymes. Proc Natl Acad Sci USA 104:11951–11956
Gundllapalli SB, Pretorius IS, Cordero Otero RR (2007) Effect of the cellulose-binding domain on the catalytic activity of a β-glucosidase from Saccharomycopsis fibuligera. J Ind Microbiol Biotechnol 34:413–421
Ha JS, Lee YM, Choi SL, Song JJ, Shin CS, Kim JH, Lee SG (2008) Thermostable β-glycosidase-CBD fusion protein for biochemical analysis of cotton scouring efficiency. J Microbiol Biotechnol 18:443–448
Holm L, Kaariainen S, Rosenstrom P, Schenkel A (2008) Searching protein structure databases with DaliLite v.3. Bioinformatics 24:2780–2781
Kumar P, Satyanarayana T (2009) Microbial glucoamylases: characteristics and applications. Crit Rev Biotechnol 29:225–255
Kumar R, Singh S, Singh OV (2008) Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. J Ind Microbiol Biotechnol 35:377–391
Kyla-Nikkilä K, Alakuijala U, Saris PE (2010) Immobilization of Lactococcus lactis to cellulosic material by cellulose-binding domain of Cellvibrio japonicus. J Appl Microbiol (in press)
Laluce C, Mattoon JR (1984) Development of rapidly fermenting strains of Saccharomyces diastaticus for direct conversion of starch and dextrins to ethanol. Appl Environ Microbiol 48:17–25
Latorre-Garcia L, Adam AC, Manzanares P, Polaina J (2005) Improving the amylolytic activity of Saccharomyces cerevisiae glucoamylase by the addition of a starch binding domain. J Biotechnol 118:167–176
Latorre-Garcia L, Adam AC, Polaina J (2008) Overexpression of the glucoamylase-encoding STA1 gene of Saccharomyces cerevisiae var. diastaticus in laboratory and industrial strains of Saccharomyces. World J Microbiol Biotechnol 24:2957–2963
Little E, Bork P, Doolittle RF (1994) Tracing the spread of fibronectin type III domains in bacterial glycohydrolases. J Mol Evol 39:631–643
Lo WS, Dranginis AM (1996) FLO11, a yeast gene related to the STA genes, encodes a novel cell surface flocculin. J Bacteriol 178:7144–7151
Machida M, Ohtsuki I, Fukui S, Yamashita I (1988) Nucleotide sequences of Saccharomycopsis fibuligera genes for extracellular β-glucosidases as expressed in Saccharomyces cerevisiae. Appl Environ Microbiol 54:3147–3155
Miettinen-Oinonen A (2007) Cellulases in the textile industry. In: Polaina J, MacCabe AP (eds) Industrial enzymes: structure, function and applications. Springer, Dordrecht, pp 51–63
Nam SW, Kim BM, Chung BH, Kang DO, Ahn JS (1994) Expression and secretion of human lipocortin-1 by promoter and signal sequence of STA1 from Sacharomyces diastaticus. Biotechnol Lett 16:897–902
Pastor FJ, Gallardo O, Sanz-Aparicio J, Díaz P (2007) Xylanases: molecular properties and applications. In: Polaina J, MacCabe AP (eds) Industrial enzymes: structure, function and applications. Springer, Dordrecht, pp 65–82
Polaina J, Wiggs MY (1983) STA10: a gene involved in the control of starch utilization by Saccharomyces. Curr Genet 7:109–112
Pozzo T, Pasten JL, Karlsson EN, Logan DT (2010) Structural and functional analyses of β-glucosidase 3B from Thermotoga neapolitana: a thermostable three-domain representative of glycoside hydrolase 3. J Mol Biol 397:724–739
Rakestraw JA, Sazinsky SL, Piatesi A, Antipov E, Wittrup KD (2009) Directed evolution of a secretory leader for the improved expression of heterologous proteins and full-length antibodies in Saccharomyces cerevisiae. Biotechnol Bioeng 103:1192–1201
Rigden DJ, Mello LV, Galperin MY (2004) The PA14 domain, a conserved all-β domain in bacterial toxins, enzymes, adhesins and signaling molecules. Trends Biochem Sci 29:335–339
Roy A, Kucukural A, Zhang Y (2010) I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5:725–738
Rubio-Texeira M, Arévalo-Rodríguez M, Lequerica JL, Polaina J (2000) Lactose utilization by Saccharomyces cerevisiae strains expressing Kluyveromyces lactis LAC genes. J Biotechnol 84:97–1006
Shen Y, Zhang Y, Ma T, Bao X, Du F, Zhuang G, Qu Y (2008) Simultaneous saccharification and fermentation of acid-pretreated corncobs with a recombinant Saccharomyces cerevisiae expressing β-glucosidase. Bioresour Technol 99:5099–5103
Sherman F (2002) Getting started with yeast. Methods Enzymol 350:3–41
Trainotti L, Spinello R, Piovan A, Spolaore S, Casadoro G (2001) β-Galactosidases with a lectin-like domain are expressed in strawberry. J Exp Bot 52:1635–1645
van Rooyen R, Hahn-Hägerdal B, La Grange DC, van Zyl WH (2005) Construction of cellobiose-growing and fermenting Saccharomyces cerevisiae strains. J Biotechnol 120:284–295
Varghese JN, Hrmova M, Fincher GB (1999) Three-dimensional structure of a barley β-D-glucan exohydrolase, a family 3 glycosyl hydrolase. Structure 7:179–190
Xu Y, Foong FC (2008) Characterization of a cellulose binding domain from Clostridium cellulovorans endoglucanase-xylanase D and its use as a fusion partner for soluble protein expression in Escherichia coli. J Biotechnol 135:319–325
Acknowledgements
This work was funded by Spanish Ministerio de Ciencia e Innnovación grant BIO2007-67708-C04-02. Leontina Gurgu was supported by a short term Collaborative Experimental Scholarship for Central & Eastern Europe from Federation of European Biochemical Societies (FEBS) and by Romanian project PNCDI II IDEI COD CNCSIS 658 No. 315/1.10.2007 awarded to Dr. Vasilica Barbu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marín-Navarro, J., Gurgu, L., Alamar, S. et al. Structural and functional analysis of hybrid enzymes generated by domain shuffling between Saccharomyces cerevisiae (var. diastaticus) Sta1 glucoamylase and Saccharomycopsis fibuligera Bgl1 β-glucosidase. Appl Microbiol Biotechnol 89, 121–130 (2011). https://doi.org/10.1007/s00253-010-2845-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2845-3