Abstract
Bacterial symbionts are increasingly recognised as mediators of ecologically important traits of their animal hosts, with acquisition of new traits possible by uptake of novel symbionts. The entomopathogenic nematode Heterorhabditis downesi associates with two bacterial symbionts, Photorhabdus temperata subsp. temperata and P. temperata subsp. cinerea. At one intensively studied coastal dune site, P. temperata subsp. cinerea is consistently more frequently isolated than P. temperata subsp. temperata in H. downesi recovered from under the bare sand/Ammophila arrenaria of the front dunes (where harsh conditions, including drought, prevail). This is not the case in the more permissive closed dune grassland further from the sea. No differences were detected in ITS1 (internal transcribed spacer) sequence between nematode lines carrying either of the two symbiont subspecies, nor did they differ in their ability to utilise insects from three orders. The two symbionts could be readily swapped between lines, and both were carried in equal numbers within infective juveniles. In laboratory experiments, we tested whether the symbionts differentially affected nematode survival in insect cadavers that were allowed to dry. We assessed numbers of nematode infective juveniles emerging from insects that had been infected with H. downesi carrying either symbiont subspecies and then allowed to desiccate for up to 62 days. In moist conditions, cadavers produced similar numbers of nematodes, irrespective of the symbiont subspecies present, while under desiccating conditions, P. temperata subsp. cinerea cadavers yielded more nematode progeny than P. temperata subsp. temperata cadavers. Desiccating cadavers with the same nematode isolates, carrying either one or the other symbiont subspecies, confirmed that the symbiont was responsible for differences in nematode survival. Moreover, cadavers harbouring P. temperata subsp. cinerea had a reduced rate of drying relative to cadavers harbouring P. temperata subsp. temperata. Our experiments support the hypothesis that H. downesi can extend its niche into harsher conditions by associating with P. temperata subsp. cinerea.





Similar content being viewed by others
References
Thrall PH, Hochberg ME, Burdon JJ, Bever JD (2007) Coevolution of symbiotic mutualists and parasites in a community context. Trends Ecol Evol 22:120–126
Douglas A (2009) The microbial dimension in insect nutritional ecology. Funct Ecol 23:38–47
Feldhaar H (2011) Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol Entomol 36:533–543
Duperron S, Pottier M-A, Leger N, Gaudron SM, Puillandre N, Le Prieur SP, Sigwart JD, Ravaux J, Zbinden M (2013) A tale of two chitons: is habitat specialisation linked to distinct associated bacterial communities? FEMS Microbiol Ecol 83:552–567
Oliver KM, Degnan PH, Burke GR, Moran NA (2010) Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55:247–266
Henry LM, Peccoud J, Simon J-C, Hadfield JD, Maiden MJ, Ferrari J, Godfray HCJ (2013) Horizontally transmitted symbionts and host colonization of ecological niches. Curr Biol 23:1713–1717
Chaston J, Goodrich-Blair H (2010) Common trends in mutualism revealed by model associations between invertebrates and bacteria. FEMS Microbiol Rev 34:41–58
Clarke DJ (2008) Photorhabdus: a model for the analysis of pathogenicity and mutualism. Cell Microbiol 10:2159–2167
Poinar GO (1993) Origins and phylogenetic relationships of the entomophilic rhabditids, Heterorhabditis and Steinernema. Fundam Appl Nematol 16:333–338
Martens EC, Heungens K, Goodrich-Blair H (2003) Early colonization events in the mutualistic association between Steinernema carpocapsae nematodes and Xenorhabdus nematophila bacteria. J Bacteriol 185:3147–3154
Ciche T, Ensign J (2003) For the insect pathogen Photorhabdus luminescens, which end of a nematode is out? Appl Environ Microbiol 69:1890–1897
Ciche TA, Kim KS, Kaufmann-Daszczuk B, Nguyen KCQ, Hall DH (2008) Cell invasion and matricide during Photorhabdus luminescens transmission by Heterorhabditis bacteriophora nematodes. Appl Environ Microbiol 74:2275–2287
Adams BJ, Fodor A, Koppenhofer HS, Stackebrandt E, Stock SP, Klein MG (2006) Biodiversity and systematics of nematode-bacterium entomopathogens. Biol Control 37:32–49
Akhurst R, Boemare N, Gaugler R, Kaya H (1990) Biology and taxonomy of Xenorhabdus. In: Gaugler R, Kaya HK (eds) Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, pp 75–90
Lee MM, Stock SP (2010) A multilocus approach to assessing co-evolutionary relationships between Steinernema spp. (Nematoda: Steinernematidae) and their bacterial symbionts Xenorhabdus spp. (γ − Proteobacteria: Enterobacteriaceae). Syst Parasitol 77:1–12
Noujeim E, Khater C, Pages S, Ogier J-C, Tailliez P, Hamze M, Thaler O (2011) The first record of entomopathogenic nematodes (Rhabiditiae: Steinernematidae and Heterorhabditidae) in natural ecosystems in Lebanon: a biogeographic approach in the Mediterranean region. J Invertebr Pathol 107:82–85
Emelianoff V, Le Brun N, Pagès S, Stock SP, Tailliez P, Moulia C, Sicard M (2008) Isolation and identification of entomopathogenic nematodes and their symbiotic bacteria from Hérault and Gard (Southern France). J Invertebr Pathol 98:211–217
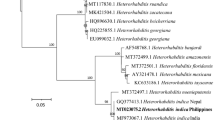
Maneesakorn P, An RS, Daneshvar H, Taylor K, Bai XD, Adams BJ, Grewal PS, Chandrapatya A (2011) Phylogenetic and cophylogenetic relationships of entomopathogenic nematodes (Heterorhabditis: Rhabditida) and their symbiotic bacteria (Photorhabdus: Enterobacteriaceae). Mol Phylogenet Evol 59:271–280
Tóth T, Lakatos T (2009) Two different bacterial symbionts of Heterorhabditis megidis and Heterorhabditis downesi inside one population. IOBC/WPRS Bull 45:395–397
Sicard M, Ferdy JB, Pages S, Le Brun N, Godelle B, Boemare N, Moulia C (2004) When mutualists are pathogens: an experimental study of the symbioses between Steinernema (entomopathogenic nematodes) and Xenorhabdus (bacteria). J Evol Biol 17:985–993
Sicard M, Ramone H, Le Brun N, Pagès S, Moulia C (2005) Specialization of the entomopathogenic nematode Steinernema scapterisci with its mutualistic Xenorhabdus symbiont. Naturwissenschaften 92:472–476
Murfin KE, Lee M-M, Klassen JL, McDonald BR, Larget B, Forst S, Stock SP, Currie CR, Goodrich-Blair H (2015) Xenorhabdus bovienii strain diversity impacts coevolution and symbiotic maintenance with Steinernema spp. nematode hosts. mBio 6:e00076-15
Gerritsen LJM, Smits PH (1993) Variation in pathogenicity of recombinations of Heterorhabditis and Xenorhabdus luminescens strains. Fundam Appl Nematol 16:367–373
Gerritsen LJM, Smits PH (1997) The influence of Photorhabdus luminescens strains and form variants on the reproduction and bacterial retention of Heterorhabditis megidis. Fundam Appl Nematol 20:317–322
Han R, Wouts W, Li L (1991) Development and virulence of Heterorhabditis spp. strains associated with different Xenorhabdus luminescens isolates. J Invertebr Pathol 58:27–32
Han RC, Ehlers RU (1998) Cultivation of axenic Heterorhabditis spp. dauer juveniles and their response to non-specific Photorhabdus luminescens food signals. Nematologica 44:425–435
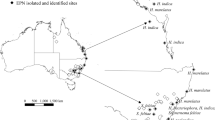
Rolston AN, Griffin CT, Downes MJ (2005) Distribution of entomopathogenic nematodes in an Irish sand dune system. Nematology 7:259–266
Rodwell JS, Birks HJB, Malloch AJC (2000) Shingle, strandline and sand-dune communities. In: Rodwell JS (ed) British plant communities, vol 5. Cambridge University Press, Cambridge, pp 113–250
Verhoeven R (2002) The structure of the microtrophic system in a development series of dune soils. Pedobiologia 46:75–89
Speight MC (1997) Invertebrates of dune and grassland. In: Jeffrey DW (ed) North Bull Island Dublin Bay—a modern coastal natural history. The Royal Dublin Society, Dublin, pp 107–111
McLachlan A, Brown AC (2006) The ecology of sandy shores. Elsevier, London
Serwe-Rodriguez J, Sonnenberg K, Appleman B, Bornstein-Forst S (2004) Effects of host desiccation on development, survival, and infectivity of entomopathogenic nematode Steinernema carpocapsae. J Invertebr Pathol 85:175–181
Koppenhöfer AM, Baur ME, Stock SP, Choo HY, Chinnasri B, Kaya HK (1997) Survival of entomopathogenic nematodes within host cadavers in dry soil. Appl Soil Ecol 6:231–240
Spence KO, Stevens GN, Arimoto H, Ruiz-Vega J, Kaya HK, Lewis EE (2011) Effect of insect cadaver desiccation and soil water potential during rehydration on entomopathogenic nematode (Rhabditida: Steinernematidae and Heterorhabditidae) production and virulence. J Invertebr Pathol 106:268–273
Lewis EE, Shapiro-Ilan DI (2002) Host cadavers protect entomopathogenic nematodes during freezing. J Invertebr Pathol 81:25–32
Webster JM, Chen G, Hu K, Li J (2002) Bacterial metabolites. In: Gaugler R (ed) Entomopathogenic nematology. CABI Publishing, Wallingford, pp 99–113
Bode HB (2009) Entomopathogenic bacteria as a source of secondary metabolites. Curr Opin Chem Biol 13:224–230
McCorry M, Ryle T (2009) A management plan for North Bull Island. Dublin City Council, Dublin
Fossitt JA (2000) A guide to habitats in Ireland. Heritage Council, Kilkenny
Bedding R, Akhurst R (1975) A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica 21:109–110
Kaya HK, Stock PS (1997) Techniques in insect nematology. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic, London, pp 281–324
Woodring JL, Kaya H (1988) Steinernematid and heterorhabitid nematodes: a handbook of biology and techniques. Arkansas Agricultural Experiment Station, Fayetteville
Hoogsteen MJJ, Lantinga EA, Bakker EJ, Groot JCJ, Tittonell PA (2015) Estimating soil organic carbon through loss on ignition: effects of ignition conditions and structural water loss. Eur J Soil Sci 66:320–328
Harding DE, Ross DJ (1964) Some factors in low-temperature storage influencing mineralisable-nitrogen of soils. J Sci Food Agric 15:829–834
Vrain TC, Wakarchuk DA, Levesque AC, Hamilton RI (1992) Intraspecific rDNA restriction-fragment-length-polymorphism in the Xiphinema-americanum group. Fundam Appl Nematol 15:563–573
Tailliez P, Boemare N (2009) Phylogenetic studies with entomopathogenic bacteria with special emphasis on symbionts of entomopathogenic nematodes. In: Stock SP, Vanderberg J, Glazer I, Boemare N (eds) Insect pathogens: molecular approaches and techniques. CAB International, Wallingford, pp 131–144
Boemare N, Tailliez P (2009) Molecular approaches and techniques for the study of entomopathogenic bacteria. In: Stock SP, Vanderberg J, Glazer I, Boemare N (eds) Insect pathogens: molecular approaches and techniques. CAB International, Wallingford, pp 32–49
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Larkin MA, Blackshields G, Brown N, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Tóth T, Lakatos T (2008) Photorhabdus temperata subsp. cinerea subsp. nov., isolated from Heterorhabditis nematodes. Int J Syst Evol Microbiol 58:2579–2581
Minitab, Inc. (2015) Minitab 17 Statistical Software. State College, PA
Grewal PS, Converse V, Georgis R (1999) Influence of production and bioassay methods on infectivity of two ambush foragers (Nematoda : Steinernematidae). J Invertebr Pathol 73:40–44
Peters A (1996) The natural host range of Steinernema and Heterorhabditis spp. and their impact on insect populations. Biocontrol Sci Tech 6:389–402
Hominick WM (2002) Biogeography. In: Gaugler R (ed) Entomopathogenic nematology, vol 1. CAB International, Wallingford, pp 115–143
Ridout MS, Hinde JP, Demetrio CGB (1998) Models for count data with many zeros. Proceedings of the 19th International Biometric Conference. Cape Town, South Africa. pp 179–192
SAS Institute (2010) SAS Version 9.3. SAS Institute Inc., Cary, NC
Willis A, Folkes B, Hope-Simpson J, Yemm E (1959) Braunton Burrows: the dune system and its vegetation. J Ecol 47:249–288
Jeffrey DW (1977) Comparision of the habitats. In: Jeffrey DW (ed) North bull island Dublin Bay - a modern coastal natural history. RDS, Dublin, pp 26–31
Perry RN (1999) Desiccation survival of parasitic nematodes. Parasitology 119:S19–S30
Glazer I (2002) Survival biology. In: Gaugler R (ed) Entomopathogenic nematology. CABI, Wallingford, pp 169–187
Ferreira AS, Leitao JH, Silva IN, Pinheiro PF, Sousa SA, Ramos CG, Moreira LM (2010) Distribution of cepacian biosynthesis genes among environmental and clinical Burkholderia strains and role of cepacian exopolysaccharide in resistance to stress conditions. Appl Environ Microbiol 76:441–450
Greene C, Vadlamudi G, Newton D, Foxman B, Xi CW (2016) The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am J Infect Control 44:E65–E71
Espinal P, Marti S, Vila J (2012) Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J Hosp Infect 80:56–60
Rossi F, Potrafka RM, Pichel FG, De Philippis R (2012) The role of the exopolysaccharides in enhancing hydraulic conductivity of biological soil crusts. Soil Biol Biochem 46:33–40
Kondakova AN, Kirsheva NA, Arbatsky NP, Shaikhutdinova RZ, Shashkov AS, Ivanov SA, Anisimov AP, Knirel YA (2015) Structure of a zwitterionic O-polysaccharide from Photorhabdus temperata subsp. cinerea 3240. Carbohydr Res 407:1–4
Rinaudo M (2004) Role of substituents on the properties of some polysaccharides. Biomacromolecules 5:1155–1165
Grewal PS, Bornstein-Forst S, Burnell AM, Glazer I, Jagdale GB (2006) Physiological, genetic, and molecular mechanisms of chemoreception, thermobiosis, and anhydrobiosis in entomopathogenic nematodes. Biol Control 38:54–65
Colinet H, Renault D (2014) Dietary live yeast alters metabolic profiles, protein biosynthesis and thermal stress tolerance of Drosophila melanogaster. Comp Biochem Physiol A Mol Integr Physiol 170:6–14
Sisodia S, Singh BN (2012) Experimental evidence for nutrition regulated stress resistance in Drosophila ananassae. PLoS One 7, e46131
Andersen LH, Kristensen TN, Loeschcke V, Toft S, Mayntz D (2010) Protein and carbohydrate composition of larval food affects tolerance to thermal stress and desiccation in adult Drosophila melanogaster. J Insect Physiol 56:336–340
Qiu L, Bedding RA (2002) Characteristics of protectant synthesis of infective juveniles of Steinernema carpocapsae and importance of glycerol as a protectant for survival of the nematodes during osmotic dehydration. Comp Biochem Physiol B: Biochem Mol Biol 131:757–765
Qiu L, Lacey MJ, Bedding RA (2000) Permeability of the infective juveniles of Steinernema carpocapsae to glycerol during osmotic dehydration and its effect on biochemical adaptation and energy metabolism. Comp Biochem Physiol B: Biochem Mol Biol 125:411–419
Huiskes AHL (1979) Ammophila arenaria (L.) Link (Psamma arenaria (L.) Roem. et Schult.; Calamgrostis arenaria (L.) Roth). J Ecol 67:363–382
Půža V, Mráček Z (2005) Seasonal dynamics of entomopathogenic nematodes of the genera Steinernema and Heterorhabditis as a response to abiotic factors and abundance of insect hosts. J Invertebr Pathol 89:116–122
Lewis EE, Clarke DJ (2012) Nematode parasites and entomopathogens. In: Vega FE, Kaya HK (eds) Insect pathology, 2nd edn. Academic, San Diego, pp 395–424
Griffin CT, Joyce SA, Dix I, Burnell AM, Downes MJ (1994) Characterisation of the entomopathogenic nematode Heterorhabditis (Nematoda: Heterorhabditidae) from Ireland and Britain by molecular and cross-breeding techniques, and the occurrence of the genus in these islands. Fundam Appl Nematol 17:245–253
Griffin CT, Dix I, Joyce SA, Burnell AM, Downes MJ (1999) Isolation and characterisation of Heterorhabditis spp. (Nematoda : Heterorhabditidae) from Hungary, Estonia and Denmark. Nematology 1:321–332
Ferreira T, van Reenen CA, Endo A, Tailliez P, Pages S, Sproer C, Malan AP, Dicks LMT (2014) Photorhabdus heterorhabditis sp. nov., a symbiont of the entomopathogenic nematode Heterorhabditis zealandica. Int J Syst Evol Microbiol 64:1540–1545
Stuart RJ, Barbercheck ME, Grewal PS, Taylor RAJ, Hoy CW (2006) Population biology of entomopathogenic nematodes: concepts, issues, and models. Biol Control 38:80–102
Tailliez P, Laroui C, Ginibre N, Paule A, Pages S, Boemare N (2010) Phylogeny of Photorhabdus and Xenorhabdus based on universally conserved protein-coding sequences and implications for the taxonomy of these two genera. Proposal of new taxa: X. vietnamensis sp nov., P. luminescens subsp caribbeanensis subsp nov., P. luminescens subsp hainanensis subsp nov., P. temperata subsp khanii subsp nov., P. temperata subsp tasmaniensis subsp nov., and the reclassification of P. luminescens subsp thracensis as P. temperata subsp thracensis comb. nov. Int J Syst Evol Microbiol 60:1921–1937
Gerritsen LJM, Wiegers GL, Smits PH (1998) Pathogenicity of new combinations of Heterorhabditis spp. and Photorhabdus luminescens against Galleria mellonella and Tipula oleracea. Biol Control 13:9–15
Acknowledgments
A.M.D. Maher was funded by a doctoral fellowship from the Irish Research Council for Science, Engineering and Technology (IRCSET); M. Asaiyah was funded by a Postgraduate Scholarship from the Ministry of Higher Education and Scientific Research in Lybia. We are grateful to Dr. David Fitzpatrick for advice on bioinformatics and to Prof. M.J. Downes and the anonymous referees for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sequences
GenBank accession numbers for Heterorhabditis downesi: KU573057, KU573058, KU573059, KU573060, KU573061, KU573062
Photorhabdus temperata subspecies temperata: KU559326, KU559327, KU559328
Photorhabdus temperata subspecies cinerea: KU559323, KU559324, KU559325
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 134 kb)
Rights and permissions
About this article
Cite this article
Maher, A.M.D., Asaiyah, M.A.M., Brophy, C. et al. An Entomopathogenic Nematode Extends Its Niche by Associating with Different Symbionts. Microb Ecol 73, 211–223 (2017). https://doi.org/10.1007/s00248-016-0829-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0829-2