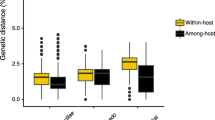
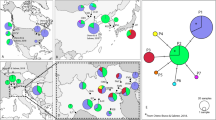
Abstract
Nematodes of the genus Steinernema Travassos, 1927 (Nematoda: Steinernematidae) and their associated bacteria, Xenorhabdus spp. (γ-Proteobacteria), are an emergent model of terrestrial animal-microbe symbiosis. Interest in this association initially arose out of their potential as biocontrol agents against insect pests, but, despite advances in their field application and the growing popularity of this model system, relatively little has been published to uncover the evolutionary facets of this beneficial partnership. This study adds to the body of knowledge regarding nematode-bacteria symbiosis by proposing a possible scenario for their historical association in the form of a cophylogenetic hypothesis. Topological and likelihood based testing methods were employed to reconstruct a history of association between 30 host-symbiont pairs and to gauge the level of similarity between their inferred phylogenetic patterns.


Similar content being viewed by others
References
Akhurst, R. J. (1980). Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. Journal of General Microbiology, 121, 303–309.
Akhurst, R. J. (1982). Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. Journal of General Microbiology, 128, 3061–3065.
Akhurst, R. J., & Boemare, N. E. (1990). Biology and taxonomy of Xenorhabdus. In: Gaugler, R. & Kaya, H. K. (Eds) Entomopathogenic nematodes in biological control. Boca Raton: CRC Press, pp. 75–90.
Baumann, L., & Baumann, P. (2005). Cospeciation between the primary endosymbionts of mealybugs and their hosts. Current Microbiology, 50, 84–87.
Boemare, N. E. (2002). Biology, taxonomy, and systematics of Photorhabdus and Xenorhabdus. In: Gaugler, R. (Ed.) Entomopathogenic nematology. Wallingford: CABI Publishing, pp. 37–79.
Chen, G., Dunphy, G. B., & Webster, J. M. (1994). Antimycotic activity of two Xenorhadus species and Photorhabdus luminescens, bacteria associated with the nematodes Steinernema species and Heterorhabditis megidis. Biological Control, 4, 339–348.
Clark, M. A., Moran, N. A., Baumann, P., & Wernegreen, J. J. (2000). Cospeciation betwen bacterial endosymbionts (Buchnera) and a recent radiaton of aphids (Uroleucon) and pitfalls of testing for phylogenetic congruence. Evolution, 54, 517–525.
Cowles, C. E., & Goodrich-Blair, H. (2004). Characterization of lipoprotein, nilC, required by Xenorhabdus nematophila for mutualism with its nematode host. Molecular Microbiology, 54, 464–477.
Cowles, C. E., & Goodrich-Blair, H. (2006). nilR is necessary for coordinate repression of Xenorhabdus nematophila mutualism genes. Molecular Microbiology, 62, 760–771.
Cowles, C. E., & Goodrich-Blair, H. (2008). The Xenorhabdus nematophila nilABC genes confer the ability of Xenorhabdus spp. to colonize Steinernema carpocapsae nematodes. Journal of Bacteriology, 190, 4121–4128.
Cowles, K. N., Cowles, C. E., Martens, E. C., & Goodrich-Blair, H. (2007). The global regulator Lrp contributes to both beneficial and pathogenic microbe-host interactions in Xenorhabdus nematophila. Cellular Microbiology, 9, 1311–1323.
Degnan, P. H., Lazarus, A. B., Brock, C. D., & Wernegreen, J. T. (2004). Host-symbiont stability and fast evolutionary rates in an ant-bacterium association: cospeciation of Camponotus species and their endosymbionts, Candidatus and Blochmannia. Systematic Biology, 53, 95–110.
Forst, S., & Clarke, D. J. (2002) Nematode-bacterium symbiosis. In: Gaugler, R. (Ed.) Entomopathogenic Nematology. Wallingford: CABI Publishing, pp. 57–77.
Hafner, M. S., & Nadler, S. A. (1988). Phylogenetic trees support the co-evolution of parasites and their hosts. Nature, 332, 258–259.
Heungens, K., Cowles, C. E., & Goodrich-Blair, H. (2002). Identification of Xenorhabdus nematophila genes required for mutualistic colonization of Steinernema carpocapsae nematodes. Molecular Microbiology, 45, 1337–1353.
Huelsenbeck, J. P., & Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogeny. Bioinformatics, 17, 754–755.
Kaya, H. K., & Gaugler, R. (1993). Entomopathogenic nematodes. Annual Revues of Entomology, 38, 181–206.
Kaya, H. K., & Stock, S. P. (1997). Techniques in insect nematology. In: Lacey, L. A. (Ed.) Manual of techniques in insect pathology. San Diego: Academic Press, pp. 281–324.
Lee, M. M., & Stock, S. P. (2010). A multigene approach for assessing evolutionary relationships of Xenorhabdus spp. (Gamma-Proteobacteria), the bacterial symbionts of entomopathogenic Steinernema nematodes. Journal of Invertebrate Pathology, 104, 67–74.
Liu, J., Berry, R., Poinar, G., & Moldenke, A. (1997). Phylogeny of Photorhabdus and Xenorhabdus species and strains as determined by comparison of partial 16S rRNA gene sequences. International Journal of Systematic Bacteriology, 47, 948–951.
Maddison, W. P., & Maddison, D. R. (2007). Mesquite: a modular system for evolutionary analysis. Version 2.01. http://mesquiteproject.org.
Maloy, S. R. (1990). Experimental techniques in bacterial genetics. Boston: Jones and Bartlett, 180 pp.
Merkle, D., & Middendorf, M. (2005). Reconstruction of cophylogenetic history of related phylogenetic trees with divergence timing information. Theoretical Biosciences, 123, 277–299.
Miller, J. H. (1972). Experiments in molecular genetics. Cold Spring Harbor: Cold Spring Harbor Laboratory Press, 468 pp.
Moran, N. A., Tran, P., & Gerardo, N. A. (2005). Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial phylum Bacteroidetes. Applied Environmental Microbiology, 71, 8802–8810.
Nadler, S. A., Bolotin, E., & Stock, S. P. (2006). Phylogenetic relationships of Steinernema (Cephalobina, Steinernematidae) based on nuclear, mitochondrial, and morphological data. Systematic Parasitology, 64, 159–179.
Nishiguchi, M. K., Ruby, E. G., & McFall-Ngai, M. J. (1998). Competitive dominance among strains of luminous bacteria provides an unusual form of evidence of parallel evolution in sepiolid squid-Vibrio symbioses. Applied Environmental Microbiology, 64, 3209–3213.
Nylander, J. A. A. (2004). MrModeltest v2.3. Program distributed by the author. Evolutionary Biology Centre, Uppsala University.
Page, R. D. M., & Charleston, M. A. (1998). Trees within trees: phylogeny and historical associations. TREE, 13, 356–359.
Paterson, A. M., Palma, R. L., & Gray, R. D. (2002). Drowning on arrival, missing the boat, and x-events: how likely are sorting events? In: Page, R. D. M. (Ed.) Tangled trees: phylogeny, cospeciation, and co-evolution. Chicago: The University of Chicago Press, pp. 287–309.
Peek, A., Feldman, R. A., Lutz, R. A., & Vrijenhoek, R. C. (1998). Cospeciation of chemoautotrophic bacteria and deep sea clams. Proceedings of the National Academy of Science, 95, 9962–9966.
Půzǎ, V., & Mráček, Z. (2009). Mixed infection of Galleria mellonella with two entomopathogenic nematodes (Nematoda: Rhabditida) species: Steinernema affine benefits from the presence of Steinernema kraussei. Journal of Invertebrate Pathology, 102, 40–43.
Refregier, G., Le Gac, M., Jabbour, F., Widmer, A., Shykoff, J. A., Yockteng, R., Hood, M., & Giraud, T. (2008). Cophylogeny of the anther smut fungi and their caryophyllaceous hosts: prevalence of host shifts and importance of delimiting parasite species for inferring cospeciation. BMC Evolutionary Biology, 8, 100.
Sergeant, M., Baxtre, L., Jarrett, P., Shaw, E., Ousley, M., Winstanley, C., & Morgan, J. A. (2006). Identification, typing and insecticidal activitiy of Xenorhabdus isolates from entomopathogenic nematodes in United Kingdom soil and characterization of xpt toxin loci. Applied Environmental Microbiology, 72, 5895–5907.
Shimodaira, H., & Hasegawa, M. (1999). Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Molecular Biology and Evolution, 16, 1114–1116.
Sicard, M., Ferdy, J. B., Pagès, S., Le Brun, N., Godelle, B., Boemare, N., & Moulia, C. (2004). When mutualists are pathogens: an experimental study of the symbioses between Steinernema (entomopathogenic nematodes) and Xenorhabdus (bacteria). Journal of Evolutionary Biology, 17, 985–993.
Sicard, M., Hinsinger, J., LeBrun, N., Pagès, S., Boemare, N., & Moulia, C. (2006). Interspecific competition between entomopathogenic nematodes (Steinernema) is modified by their bacterial symbionts (Xenorhabdus). BMC Evolutionary Biology, 6, 68–78.
Snyder, H., Stock, S. P., Kim, S. K., Flores-Lara, Y., & Forst, S. (2007). New insights into the colonization and release processes of Xenorhabdus nematophila and the morphology and ultrastructure of the bacterial recepticle of its nematode host, Steinernema carpocapsae. Applied Environmental Microbiology, 73, 5338–5346.
Spiridonov, S. E., Reid, A. P., Podrucka, K., Subbotin, S. A., & Moens, M. (2004). Phylogenetic relationships within the genus Steinernema (Nematoda: Rhabditida) as inferred from analysis of sequences of the ITS-1-5.8S-ITS2 region of rDNA and morphological features. Nematology, 6, 547–566.
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22, 2688–2690.
Swofford, D. L. (2002). PAUP* Phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland, Massachusetts: Sinauer Associates Inc.
Tailliez, P., Pagès, S., Ginibre, N., & Boemare, N. E. (2006). New insight into diversity in the genus Xenorhabdus, including the description of ten novel species. International Journal of Systematic and Evolutionary Microbiology, 56, 2805–2818.
Tailliez, P., Laroui, C., Ginibre, N., Paule, A., Pagès, S., & Boemare, N. E. (2010). Phylogeny of Photorhabdus and Xenorhabdus based on universally conserved protein-coding sequences and implications for the taxonomy of these two genera. International Journal of Systematic and Evolutionary Microbiology (in press).
Thao, M. L., Moran, N. A., Abbot, P., Brennan, E. B., Burkhardt, D. H., & Baumann, P. (2000). Cospeciation of psyllids and their primary prokaryotic endosymbionts. Applied Environmental Microbiology, 66, 2898–2905.
Thompson, J. D., Gibston, T. J., Plewniak, F., Jeanmougin, F., & Higgins, D. G. (1997). The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25, 4876–4882.
Thompson, J. N. (2005). The geographic mosaic of co-evolution. Chicago: University of Chicago Press, 443 pp.
Vivas, E. I., & Goodrich-Blair, H. (2001). Xenorhabdus nematophilus as a model for host-bacterium interactions: rpoS is necessary for mutualism with nematodes. Journal of Bacteriology, 183, 4687–4693.
Xu, J., & Hurlbert, R. E. (1990). Toxicity of irradiated media for Xenorhabdus spp. Applied Environmental Microbiology, 56, 815–818.
Zwickl, D. J. (2006). Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under maximum likelihood criterion. PhD Dissertation, The University of Texas at Austin, 125 pp.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, MM., Stock, S.P. A multilocus approach to assessing co-evolutionary relationships between Steinernema spp. (Nematoda: Steinernematidae) and their bacterial symbionts Xenorhabdus spp. (γ-Proteobacteria: Enterobacteriaceae). Syst Parasitol 77, 1–12 (2010). https://doi.org/10.1007/s11230-010-9256-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-010-9256-9