Abstract
Objective
The objective of this study was to determine the influence of CYP2C9, VKORC1, CYP4F2, and GGCX genetic polymorphisms on mean daily dose of acenocoumarol in South Indian patients and to develop a new pharmacogenetic algorithm based on clinical and genetic factors.
Methods
Patients receiving acenocoumarol maintenance therapy (n = 230) were included in the study. Single nucleotide polymorphisms (SNP) of CYP2C9, VKORC1, CYP4F2, and GGCX were genotyped by real-time polymerase chain reaction (RT-PCR) method.
Results
The mean daily acenocoumarol maintenance dose was found to be 3.7 ± 2.3 (SD) mg/day. The CYP2C9 *1*2, CYP2C9 *1*3, and CYP2C9 *2*3 variant genotypes significantly reduced the dose by 56.7 % (2.0 mg), 67.6 % (1.6 mg), and 70.3 % (1.5 mg) than wild-type carriers 4.1 mg, p < 0.0001. The genetic variants of CYP2C9 and GGCX (rs11676382) were found to be associated with lower acenocoumarol dose, whereas CYP4F2 (rs2108622) was associated with higher doses. Age, body mass index (BMI), variation of CYP2C9, VKORC1, CYP4F2, and GGCX were the major determinants of acenocoumarol maintenance dose, accounting for 61.8 % of its variability (adjusted r 2 = 0.615, p < 0.0001). Among the VKORC1 variants, rs9923231 alone contributed up to 28.6 % of the acenocoumarol dose variation.
Conclusion
VKORC1 rs9923231 polymorphism had the highest impact on acenocoumarol daily dose. A new pharmacogenetic algorithm was established to determine the acenocoumarol dose in South Indian population.

Similar content being viewed by others
References
Buller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE (2004) Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:401S–428S
Salem DN, Stein PD, Al-Ahmad A, Bussey HI, Horstkotte D, Miller N et al (2004) Antithrombotic therapy in valvular heart disease–native and prosthetic: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:457S–482S
Singer DE, Albers GW, Dalen JE, Go AS, Halperin JL, Manning WJ (2004) Antithrombotic therapy in atrial fibrillation: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:429S–456S
Horowitz B, Minor P, Morgenthaler JJ, Burnouf T, McIntosh R, Padilla A et al (2004) WHO Expert Committee on Biological Standardization. World Health Organ Tech Rep 924:1–232, backcover
James AH, Britt RP, Raskino CL, Thompson SG (1992) Factors affecting the maintenance dose of warfarin. J Clin Pathol 45:704–706
Verde Z, Santiago C, Valle B, Fernandez-Santander A, Bandres F, Calvo E et al (2009) Pharmacogenetics of acenocoumarol: CYP2C9 *2 and VKORC1 c.-1639G > A, 497C > G, 1173C > T, and 3730G > A variants influence drug dose in anticoagulated patients. Thromb Haemost 101:591–593
Carcas AJ, Borobia AM, Velasco M, Bad-Santos F, Diaz MQ, Fernandez-Capitan C et al (2012) Efficiency and effectiveness of the use of an acenocoumarol pharmacogenetic dosing algorithm versus usual care in patients with venous thromboembolic disease initiating oral anticoagulation: study protocol for a randomized controlled trial. Trials 13:239
Verhoef TI, Redekop WK, Buikema MM, Schalekamp T, van der Meer FJ, le CS et al (2012) Long-term anticoagulant effects of the CYP2C9 and VKORC1 genotypes in acenocoumarol users. J Thromb Haemost 10:606–614
Li T, Chang CY, Jin DY, Lin PJ, Khvorova A, Stafford DW (2004) Identification of the gene for vitamin K epoxide reductase. Nature 427:541–544
Liu Y, Zhong SL, Tan HH, Yang M, Fei HW, Yu XY et al (2011) Impact of CYP2C9 and VKORC1 polymorphism on warfarin response during initiation of therapy. Zhonghua Xin Xue Guan Bing Za Zhi 39:929–935
Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL et al (2005) Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 352:2285–2293
Perez-Andreu V, Roldan V, Anton AI, Garcia-Barbera N, Corral J, Vicente V et al (2009) Pharmacogenetic relevance of CYP4F2 V433M polymorphism on acenocoumarol therapy. Blood 113:4977–4979
King CR, Deych E, Milligan P, Eby C, Lenzini P, Grice G et al (2010) Gamma-glutamyl carboxylase and its influence on warfarin dose. Thromb Haemost 104:750–754
Gage BF, Eby C, Milligan PE, Banet GA, Duncan JR, McLeod HL (2004) Use of pharmacogenetics and clinical factors to predict the maintenance dose of warfarin. Thromb Haemost 91:87–94
Rathore SS, Agarwal SK, Pande S, Singh SK, Mittal T, Mittal B (2012) Therapeutic dosing of acenocoumarol: proposal of a population specific pharmacogenetic dosing algorithm and its validation in north Indians. PLoS One 7:e37844
Tham LS, Goh BC, Nafziger A, Guo JY, Wang LZ, Soong R et al (2006) A warfarin-dosing model in Asians that uses single-nucleotide polymorphisms in vitamin K epoxide reductase complex and cytochrome P450 2C9. Clin Pharmacol Ther 80:346–355
Verde Z, Ruiz JR, Santiago C, Valle B, Bandres F, Calvo E et al (2010) A novel, single algorithm approach to predict acenocoumarol dose based on CYP2C9 and VKORC1 allele variants. PLoS One 5:e11210
Takahashi H, Wilkinson GR, Nutescu EA, Morita T, Ritchie MD, Scordo MG et al (2006) Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African-Americans. Pharmacogenet Genomics 16:101–110
Dang MT, Hambleton J, Kayser SR (2005) The influence of ethnicity on warfarin dosage requirement. Ann Pharmacother 39:1008–1012
Jose R, Chandrasekaran A, Sam SS, Gerard N, Chanolean S, Abraham BK et al (2005) CYP2C9 and CYP2C19 genetic polymorphisms: frequencies in the south Indian population. Fundam Clin Pharmacol 19:101–105
Xie HG, Prasad HC, Kim RB, Stein CM (2002) CYP2C9 allelic variants: ethnic distribution and functional significance. Adv Drug Deliv Rev 54:1257–1270
Scibona P, Redal MA, Garfi LG, Arbelbide J, Argibay PF, Belloso WH (2012) Prevalence of CYP2C9 and VKORC1 alleles in the Argentine population and implications for prescribing dosages of anticoagulants. Genet Mol Res 11:70–76
Reich D, Thangaraj K, Patterson N, Price AL, Singh L (2009) Reconstructing Indian population history. Nature 461:489–494
Krishnakumar D, Gurusamy U, Dhandapani K, Surendiran A, Baghel R, Kukreti R et al (2012) Genetic polymorphisms of drug-metabolizing phase I enzymes CYP2E1, CYP2A6 and CYP3A5 in South Indian population. Fundam Clin Pharmacol 26:295–306
Naveen AT, Adithan C, Soya SS, Gerard N, Krishnamoorthy R (2006) CYP2D6 genetic polymorphism in South Indian populations. Biol Pharm Bull 29:1655–1658
Kumar DK, Shewade DG, Surendiran A, Adithan C (2013) Genetic variation and haplotype structure of the gene Vitamin K epoxide reductase complex, subunit 1 in the Tamilian population. J Pharmacol Pharmacother 4:53–58
Barrett JC (2009) Haploview: Visualization and analysis of SNP genotype data. Cold Spring Harb Protoc 2009: db
Gurwitz JH, Avorn J, Ross-Degnan D, Choodnovskiy I, Ansell J (1992) Aging and the anticoagulant response to warfarin therapy. Ann Intern Med 116:901–904
Yoshizawa M, Hayashi H, Tashiro Y, Sakawa S, Moriwaki H, Akimoto T et al (2009) Effect of VKORC1(rs9923231) polymorphism, body weight, age, and serum albumin alterations on warfarin response in Japanese patients. Thromb Res 124:161–166
Gan GG, Teh A, Goh KY, Chong HT, Pang KW (2003) Racial background is a determinant factor in the maintenance dosage of warfarin. Int J Hematol 78:84–86
Yuan HY, Chen JJ, Lee MT, Wung JC, Chen YF, Charng MJ et al (2005) A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum Mol Genet 14:1745–1751
Bodin L, Verstuyft C, Tregouet DA, Robert A, Dubert L, Funck-Brentano C et al (2005) Cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genotypes as determinants of acenocoumarol sensitivity. Blood 106:135–140
Cini M, Legnani C, Cosmi B, Guazzaloca G, Valdre L, Frascaro M et al (2012) A new warfarin dosing algorithm including VKORC1 3730 G > A polymorphism: comparison with results obtained by other published algorithms. Eur J Clin Pharmacol 68:1167–1174
Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP et al (2007) Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 116:2563–2570
Cavallari LH, Momary KM, Patel SR, Shapiro NL, Nutescu E, Viana MA (2011) Pharmacogenomics of warfarin dose requirements in Hispanics. Blood Cells Mol Dis 46:147–150
Gan GG, Phipps ME, Lee MM, Lu LS, Subramaniam RY, Bee PC et al (2011) Contribution of VKORC1 and CYP2C9 polymorphisms in the interethnic variability of warfarin dose in Malaysian populations. Ann Hematol 90:635–641
Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, Lee MT et al (2009) Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med 360:753–764
Ohno M, Yamamoto A, Ono A, Miura G, Funamoto M, Takemoto Y et al (2009) Influence of clinical and genetic factors on warfarin dose requirements among Japanese patients. Eur J Clin Pharmacol 65:1097–1103
Pavani A, Naushad SM, Mishra RC, Malempati AR, Pinjala R, Kumar TR et al (2012) Retrospective evidence for clinical validity of expanded genetic model in warfarin dose optimization in a South Indian population. Pharmacogenomics 13:869–878
Shahin MH, Khalifa SI, Gong Y, Hammad LN, Sallam MT, El SM et al (2011) Genetic and nongenetic factors associated with warfarin dose requirements in Egyptian patients. Pharmacogenet Genomics 21:130–135
Shrif NE, Won HH, Lee ST, Park JH, Kim KK, Kim MJ et al (2011) Evaluation of the effects of VKORC1 polymorphisms and haplotypes, CYP2C9 genotypes, and clinical factors on warfarin response in Sudanese patients. Eur J Clin Pharmacol 67:1119–1130
Kumar DK, Shewade DG, Loriot MA, Beaune P, Balachander J, Sai Chandran BV et al (2014) Effect of CYP2C9, VKORC1, CYP4F2 and GGCX genetic variants on warfarin maintenance dose and explicating a new pharmacogenetic algorithm in South Indian population. Eur J Clin Pharmacol 70:47–56
Cerezo-Manchado JJ, Rosafalco M, Anton AI, Perez-Andreu V, Garcia-Barbera N, Martinez AB et al (2013) Creating a genotype-based dosing algorithm for acenocoumarol steady dose. Thromb Haemost 109:146–153
van Schie RM, Babajeff AM, Schalekamp T, Wessels JA, Le CS, De BA et al (2012) An evaluation of gene-gene interaction between the CYP2C9 and VKORC1 genotypes affecting the anticoagulant effect of phenprocoumon and acenocoumarol. J Thromb Haemost 10:767–772
Verhoef TI, Ragia G, de Boer A, Barallon R, Kolovou G, Kolovou V et al (2013) A randomized trial of genotype- guided dosing of acenocoumarol and phenprocoumon. N Engl J Med 369:2304–2312
Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T et al (2013) A randomized trial of genotype- guided dosing of warfarin. N Engl J Med 369:2294–2303
Saraeva RB, Paskaleva ID, Doncheva E, Eap CB, Ganev VS (2007) Pharmacogenetics of acenocoumarol: CYP2C9, CYP2C19, CYP1A2, CYP3A4, CYP3A5 and ABCB1 gene polymorphisms and dose requirements. J Clin Pharm Ther 32:641–649
Teichert M, Eijgelsheim M, Rivadeneira F, Uitterlinden AG, van Schaik RH, Hofman A et al (2009) A genome-wide association study of acenocoumarol maintenance dosage. Hum Mol Genet 18:3758–3768
Acknowledgments
This study was a collaborative project between INSERM UMR775–Bases Moléculaires de la réponse aux xénobiotiques located in the Université Paris-Descartes, Paris, France and Indian council of Medical Research, New Delhi, India. It was supported by a research grant (ICMR Ref. No. 50/6/2010/BMS, dated 03/11/2010) from the ICMR, New Delhi. The technical staffs of departments of pharmacology, cardiology and CTVS are gratefully acknowledged.
Conflict of interest
All the authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
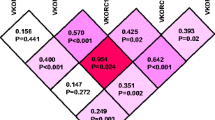
LD pattern of the haplotypes tagSNPs. The single nucleotide polymorphisms in chromosome 16 were positioned according to the order and orientation. Each of the variant is given with their specific chromosomal position and the rsID. LD pattern of the rs9923231, rs7196161, rs2884737, rs1770847, rs9934438, rs8050894, rs2359612 and rs7294 in the study population. Red and pink colors represent a very strong LD pattern (D’> 0.8) and white color represents moderate to low LD (D’<0.8 to >0.5). The D’ prime values given inside the color boxes respect to the LD between the SNPs. (DOCX 13 kb)
ESM 2
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Krishna Kumar, D., Shewade, D.G., Loriot, MA. et al. An acenocoumarol dosing algorithm exploiting clinical and genetic factors in South Indian (Dravidian) population. Eur J Clin Pharmacol 71, 173–181 (2015). https://doi.org/10.1007/s00228-014-1791-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1791-x