Abstract
Purpose
The purpose of this study is to assess the incidence of adverse drug reactions (ADR) leading to call an emergency medical dispatching centre.
Methods
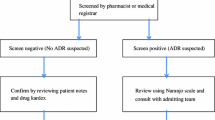
A prospective, observational, monocentric clinical study performed over a 2-year period (2011–2012) in a French prehospital emergency dispatching centre, the Service d'Aide Médicale Urgente (SAMU) covering 1,156,000 inhabitants. All adult patients (age ≥ 18) who called for any cause were included. We created an electronic trigger ‘iatrogenic event’ implemented by the dispatching physician for each suspected case of ADR, then we completed the analyses of all the cases with a chief complain represented in more than 1 % of the triggered cases. The primary outcome variable was the occurrence of any possible ADR. We then used the French method of causal relationship assessment.
Results
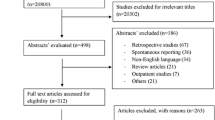
The SAMU dispatched 339,915 calls during the study. In total, 1,467 ADRs were identified, representing 0.95 % (CI 95 % 0.90–1.00 %) of cases. ADRs were as serious (SADR) in 51.06 % (CI 95 % 48.45–53.67 %) of cases. The major ADR observed was haemorrhage, (42.81 % (CI 95 % 40.62–45.00 %), n = 628) followed by allergy, hypoglycaemia, vomiting, dizziness and drowsiness. The class of drugs most frequently involved was antithrombotic (43.69 % (CI 95 % 41.45–45.93 %), n = 641), followed by insulin (17.98 % (CI 95 %:17.06–18.90 %), n = 264).
Conclusions
Emergency calls concerning ADRs were estimated as 9/1,000, and one out of two is serious.


Similar content being viewed by others
References
Queneau P, Bergmann JF (2001) Prévention de la iatrogénie évitable: quand la sécurité des malades se conjugue avec les économies de santé. Therapie 56:163–168
Lazarou J (1998) Incidence of adverse drug reactions in hospitalized patients. JAMA 279:1200–1205
Pouyanne P, Haramburu F, Imbs JL, Bégaud B (2000) Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. Br Med J 320:1036
Capuano A, Irpino A, Gallo M, Ferrante L, Illiano ML, Rinaldi B et al (2009) Regional surveillance of emergency-department visits for outpatient adverse drug events. Eur J Clin Pharmacol 65:721–728
Malhotra S, Jain S, Pandhi P (2001) Drug-related visits to the medical emergency department: a prospective study from India. Int J Clin Pharmacol Ther 39:12–18
Olivier P, Boulbés O, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M (2002) Assessing the feasibility of using an adverse drug reaction preventability scale in clinical practice: a study in a French Emergency Department. Drug Saf 25:1035–1044
Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M (2009) Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department: a prospective study. Drugs Aging 26:475–482
Begaud B, Evreux JC, Jouglard J, Lagier G (1985) Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie 40:111–118
Bousquet C, Lagier G, Lillo-Le Louët A, Le Beller C, Venot A, Jaulent MC (2005) Appraisal of the MedDRA conceptual structure for describing and grouping adverse drug reactions. Drug Saf 28:19–34
Edwards IR, Aronson JK (2000) Adverse drug reactions: definitions, diagnosis, and management. Lancet 356:1255–1259
Norwegian Institute of Public Health (2009) WHO Collaborating Centre for Drug Statistics Methodology, 2009th edn. Guidelines for ATC classification and DDD assignment 2010, Oslo
Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P et al (2006) Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 63:136–147
Hernandez-Salazar A, Rosales SP, Rangel-Frausto S, Criollo E, Archer-Dubon C, Orozco-Topete R (2006) Epidemiology of adverse cutaneous drug reactions. A prospective study in hospitalized patients. Arch Med Res 37:899–902
Patel H, Bell D, Molokhia M, Srishanmuganathan J, Patel M, Car J et al (2007) Trends in hospital admissions for adverse drug reactions in England: analysis of national hospital episode statistics 1998-2005. BMC Clin Pharmacol 7:9–19
Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ et al (2004) Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 329:15–19
Hafner JW, Belknap SM, Squillante MD, Bucheit KA (2002) Adverse drug events in emergency department patients. Ann Emerg Med 39:258–267
Lvovschi V, Aubrun F, Bonnet P, Bouchara A, Bendahou M, Humbert B et al (2008) Intravenous morphine titration to treat severe pain in the ED. Am J Emerg Med 26:676–682
Bigby M, Jick S, Jick H, Arndt KA (1986) Drug-induced cutaneous reactions. A report from the Boston Collaborative Drug Surveillance Program on 15, 438 consecutive inpatients, 1975-82. JAMA 256:335–363
Coordination CRPV de Bordeaux (2007) EMIR : Effets indésirables des Médicaments : Incidence et Risque, sur les hospitalisations liées à un effet indésirable médicamenteux. Available at: http://www.sante.gouv.fr/IMG/pdf/EMIR.pdf. Accessed 11 November 2013
Mutuelle de la santé et du social (2006) ENEIS study: Goals and methodology. Soins 702:53–54
Conflict of interest
The authors have no conflict of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dehours, E., Bounes, V., Bagheri, H. et al. Adverse drug reactions in an emergency medical dispatching centre. Eur J Clin Pharmacol 70, 881–887 (2014). https://doi.org/10.1007/s00228-014-1685-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1685-y