Abstract
Purpose
The aim of this study was to estimate the prevalence of mortality among patients due to adverse drug reactions that lead to hospitalisation (fatal ADRAd), to explore the heterogeneity in its estimation through subgroup analysis of study characteristics, and to identify system-organ classes involved and causative drugs for fatal ADRAd.
Methods
We identified prospective ADRAd-related studies via screening of the PubMed and Google Scholar databases with appropriate key terms. We estimated the prevalence of fatal ADRAd using a double arcsine method and explored heterogeneity using the following study characteristics: age groups, wards, study region, ADR definitions, ADR identification methods, study duration and sample size. We examined patterns of fatal ADRAd and causative drugs.
Results
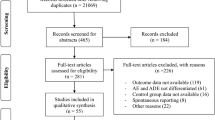
Among 312 full-text articles assessed, 49 studies satisfied the selection criteria and were included in the analysis. The mean prevalence of fatal ADRAd was 0.20% (95% CI: 0.13–0.27%; I2 = 93%). The age groups and study wards were the important heterogeneity modifiers. The mean fatal ADRAd prevalence varied from 0.01% in paediatric patients to 0.44% in the elderly. Subgroup analysis showed a higher prevalence of fatal ADRAd in intensive care units, emergency departments, multispecialty wards and whole hospitals. Computer-based monitoring systems in combination with other methods detected higher mortality. Intracranial haemorrhage, renal failure and gastrointestinal bleeding accounted for more than 50% of fatal ADRAdcases. Warfarin, aspirin, renin–angiotensin system (RAS) inhibitors and digoxin accounted for 60% of fatal ADRAd.
Conclusions
ADRAd is an important cause of mortality. Strategies targeting the safer use of warfarin, aspirin, RAS inhibitors and digoxin could reduce the large number of fatal ADRAdcases.


Similar content being viewed by others
References
Lazarou J, Pomeranz BH, Corey PN (1998) Prevalence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279(15):1200–1205
Patel H, Bell D, Molokhia M, Srishanmuganathan J, Patel M, Car J et al (2007) Trends in hospital admissions for adverse drug reactions in England: analysis of national hospital episode statistics 1998–2005. BMC Clin Pharmacol 7:9
Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M (2009) Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS One 4(2):e4439
Suh DC, Woodall BS, Shin SK, Hermes-De Santis ER (2000) Clinical and economic impact of adverse drug reactions in hospitalized patients. Ann Pharmacother 34(12):1373–1379
Del Pozzo-Magaña BR, Rieder MJ, Lazo-Langner A (2015) Quality of life in children with adverse drug reactions: a narrative and systematic review. Br J Clin Pharmacol 80(4):827–833
Patel NS, Patel TK, Patel PB, Naik VN, Tripathi CB (2017) Hospitalizations due to preventable adverse reactions-a systematic review. Eur J Clin Pharmacol 73(4):385–398
Leendertse AJ, Visser D, Egberts AC, van den Bemt PM (2010) The relationship between study characteristics and the prevalence of medication-related hospitalizations: a literature review and novel analysis. Drug Saf 33(3):233–244
Angamo MT, Chalmers L, Curtain CM, Bereznicki LR (2016) Adverse-drug-reaction-related Hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Saf 39(9):847–857
Edwards IR, Aronson JK (2000) Adverse drug reactions: definitions, diagnosis and management. Lancet 356(9237):1255–1259
Smyth RMD, Gargon E, Kirkham J, Cresswell L, Golder S, Smyth R et al (2012) Adverse drug reactions in children—a systematic review. PLoS One 7(3):e24061
Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T (2013) Meta-analysis of prevalence. J Epidemiol Community Health 67(11):974–978
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Karch FE, Lasagna L (1975) Adverse drug reactions. A critical review. JAMA 234(12):1236–1241
Aronson JK, Ferner RE (2005) Clarification of terminology in drug safety. Drug Saf 28(10):851–870
Alsbou M, Alzubiedi S, Alzobi H, Samhadanah NA, Alsaraireh Y, Alrawashdeh O et al (2015) Adverse drug reactions experience in a teaching hospital in Jordan. Int J Clin Pharm 37(6):1188–1193
Alvarez PA, Bril F, Castro V, Meiville I, Gonzalez CD, Centurion IG et al (2013) Adverse drug reactions as a reason for admission to an internal medicine ward in Argentina. Int J Risk Saf Med 25(3):185–192
Benard-Laribierea A, Miremont-Salamea G, Perault-Pochatc MC, Noizea P, Haramburua F (2015) EMIR study group on behalf of the French network of pharmacovigilance centres. Incidence of hospital admissions due to adverse drug reactions in France: the EMIR study. Fundam Clin Pharmacol 29(1):106–111
Chan M, Nicklason F, Vial JH (2001) Adverse drug events as a cause of hospital admission in the elderly. Intern Med J 31(4):199–205
Chan SL, Ang X, Sani LL, Ng HY, Winther MD, Liu JJ et al (2016) Prevalence and characteristics of adverse drug reactions at admission to hospital: a prospective observational study. Br J Clin Pharmacol 82(6):1636–1646
Davies EC, Green CF, Mottram DR, Rowe PH, Pirmohamed M (2010) Emergency re-admissions to hospital due to adverse drug reactions within 1 year of the index admission. Br J Clin Pharmacol 70(5):749–755
Dormann H, Criegee-Rieck M, Neubert A, Egger T, Geise A, Krebs S et al (2003) Lack of awareness of community-acquired adverse drug reactions upon hospital admission dimensions and consequences of a dilemma. Drug Saf 26(5):353–362
Dormann H, Neubert A, Criegee-Rieck M, Egger T, Radespiel-Troger M, Azaz-Livshits T et al (2004) Readmissions and adverse drug reactions in internal medicine: the economic impact. J Intern Med 255(6):653–663
Doshi MS, Patel PP, Shah SP, Dikshit RK (2012) Intensive monitoring of adverse drug reactions in hospitalized patients of two medical units at a tertiary care teaching hospital. J Pharmacol Pharmacother 3(4):308–313
Fattahi F, Pourpak Z, Moin M, Kazemnejad A, Khotaei GT, Mamishi S et al (2005) Adverse drug reactions in hospitalized children in a department of infectious diseases. J Clin Pharmacol 45(11):1313–1318
Gallagher RM, Bird KA, Mason JR, Peak M, Williamson PR, Nunn AJ et al (2011) Adverse drug reactions causing admission to a paediatric hospital: a pilot study. J Clin Pharm Ther 36(2):194–199
Gallagher RM, Mason JR, Bird KA, Kirkham JJ, Peak M, Williamson PR et al (2012) Adverse drug reactions causing admission to a Paediatric hospital. PLoS One 7(12):e50127
Gholami K, Babaie F, Shalviri G, Javadi MR, Faghihi T (2015) Pediatric hospital admission due to adverse drug reactions: report from a tertiary center. J Res Pharm Pract 4(4):212–215
Girgin MC, Yanturali S, Arici MA, Çolak Oray N, Doylan Ö, Demiral Y et al (2016) Emergency department visits caused by adverse drug reactions: results of a Turkish university hospital. Turk J Med Sci 46(4):945–952
Green CF, Mottram DR, Rowe PH, Pirmohamed M (2000) Adverse drug reactions as a cause of admission to an acute medical assessment unit: a pilot study. J Clin Pharm Ther 25(5):355–361
Grenouillet-Delacre M, Verdoux H, Moore N, Haramburu F, Miremont-Salamé G, Etienne G et al (2007) Life-threatening adverse drug reactions at admission to medical intensive care: a prospective study in a teaching hospital. Intensive Care Med 33(12):2150–2157
Haffner S, von Laue N, Wirth S, Thürmann PA (2005) Detecting adverse drug reactions on paediatric wards: intensified surveillance versus computerised screening of laboratory values. Drug Saf 28(5):453–464
Harugeri A, Parthasarathi G, Ramesh M, Guido S, Basavanagowdappa H (2011) Frequency and nature of adverse drug reactions in elderly in-patients of two Indian medical college hospitals. J Postgrad Med 57(3):189–195
Hofer-Dueckelmann C, Prinz E, Beindl W, Szymanski J, Fellhofer G, Pichler M et al (2011) Adverse drug reactions (ADRs) associated with hospital admissions - elderly female patients are at highest risk. Int J Clin Pharmacol Ther 49(10):577–586
Hohl CM, Zed PJ, Brubacher JR, Abu-Laban RB, Loewen PS, Purssell RA (2010) Do emergency physicians attribute drug-related emergency department visits to medication-related problems? Ann Emerg Med 55(6):493–502
Hopf Y, Watson M, Williams D (2008) Adverse-drug-reaction related admissions to a hospital in Scotland. Pharm World Sci 30(6):854–862
Lamabadusuriya SP, Sathiadas G (2003) Adverse drug reactions in children requiring hospital admission. Ceylon Med J 48(3):86–87
Langerová P, Vrtal J, Urbánek K (2014) Adverse drug reactions causing hospital admissions in childhood: a prospective, observational, single-centre study. Basic Clin Pharmacol Toxicol 115(6):560–564
Laroche ML, Charmes JP, Nouaille Y, Picard N, Merle L (2007) Is inappropriate medication use a major cause of adverse drug reactions in the elderly? Br J Clin Pharmacol 63(2):177–186
Ma J, Wang Y, Gao M, Meng Q, Liu J (2012) Adverse drug reactions as the cause of emergency department admission of patients aged 80 years and older. Eur J Intern Med 23(6):e162–e163
Malhotra S, Jain S, Pandhi P (2001) Drug-related visits to the medical emergency department: a prospective study from India. Int J Clin Pharmacol Ther 39(1):12–18
Mannesse CK, Derkx FH, de Ridder MA, Man in't Veld AJ, van der Cammen TJ (2000) Contribution of adverse drug reactions to hospital admission of older patients. Age Ageing 29(1):35–39
Ganeva M, Gancheva T, Troeva J, Gancheva D, Hristakieva E (2016) A study of adverse drug reactions in hospitalized patients in relation to age. Eur J Clin Pharm 18(3):154–162
Mehta U, Durrheim DN, Blockman M, Kredo T, Gounden R, Barnes KI (2008) Adverse drug reactions in adult medical inpatients in a south African hospital serving a community with a high HIV/AIDS prevalence: prospective observational study. Br J Clin Pharmacol 65(3):396–406
Mjörndal T, Boman MD, Hägg S, Bäckström M, Wiholm BE, Wahlin A et al (2002) Adverse drug reactions as a cause for admissions to a department of internal medicine. Pharmacoepidemiol Drug Saf 11(1):65–72
Mouton JP, Njuguna C, Kramer N, Stewart A, Mehta U, Blockman M et al (2016) Adverse drug reactions causing admission to medical wards: a cross-sectional survey at 4 hospitals in South Africa. Medicine (Baltimore) 95(19):e3437
Olivier P, Boulbés O, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M (2002) Assessing the feasibility of using an adverse drug reaction preventability scale in clinical practice a study in a French emergency department. Drug Saf 25(14):1035–1044
Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M (2009) Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department: a prospective survey. Drugs Aging 26(6):475–482
Onder G, Pedone C, Landi F, Cesari M, Della Vedova C, Bernabei R et al (2002) Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the elderly (GIFA). J Am Geriatr Soc 50(12):1962–1968
Oshikoya KA, Chukwura H, Njokanma OF, Senbanjo IO, Ojo I (2011) Incidence and cost estimate of treating pediatric adverse drug reactions in Lagos, Nigeria. Sao Paulo Med J 129(3):153–164
Patel KJ, Kedia MS, Bajpai D, Mehta SS, Kshirsagar NA, Gogtay NJ (2007) Evaluation of the prevalence and economic burden of adverse drug reactions presenting to the medical emergency department of a tertiary referral centre: a prospective study. BMC Clin Pharmacol 7:8
Pedrós C, Quintana B, Rebolledo M, Porta N, Vallano A, Arnau JM (2014) Prevalence, risk factors and main features of adverse drug reactions leading to hospital admission. Eur J Clin Pharmacol 70(3):361–367
Pedrós C, Formiga F, Corbella X, Arnau JM (2016) Adverse drug reactions leading to urgent hospital admission in an elderly population: prevalence and main features. Eur J Clin Pharmacol 72(2):219–226
Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ et al (2004) Adverse drug reactions as cause of admission to hospital: prospective analysis of 18820 patients. BMJ 329(7456):15–19
Posthumus AAG, Alingh CCW, Zwaan CCM, van Grootheest KK, Hanff LL, Witjes BB et al (2012) Adverse drug reaction related admissions in paediatrics, a prospective single-centre study. BMJ Open 2(4):e000934
Pouyanne P, Haramburu F, Imbs JL, Bégaud B (2000) Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. French pharmacovigilance Centres. BMJ 320(7241):1036
Rottenkolber D, Schmiedl S, Rottenkolber M, Farker K, Saljé K, Mueller S et al (2011) Adverse drug reactions in Germany: direct costs of internal medicine hospitalizations. Pharmacoepidemiol Drug Saf 20(6):626–634
Rivkin A (2007) Admissions to a medical intensive care unit related to adverse drug reactions. Am J Health-Syst Pharm 64(17):1840–1843
Schneeweiss S, Hasford J, Göttler M, Hoffmann A, Riethling AK, Avorn J (2002) Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population-based study. Eur J Clin Pharmacol 58(4):285–291
Schwake L, Wollenschläger I, Stremmel W, Encke J (2009) Adverse drug reactions and deliberate self-poisoning as cause of admission to the intensive care unit: a 1-year prospective observational cohort study. Intensive Care Med 35(2):266–274
Sivasankaran P, Gupta M, Satyanarayan RB, Durai R (2016) Pattern of adverse drug reactions in a Govt. District Headquarters Hospital in Tamilnadu, India. Ind J Pharm Pract 9(1):32–36
Tumwikirize WA, Ogwal-Okeng JW, Vernby A, Anokbonggo WW, Gustafsson LL, Lundborg SC (2011) Adverse drug reactions in patients admitted on internal medicine wards in a district and regional hospital in Uganda. Afr Health Sci 11(1):72–78
van der Hooft CS, Dieleman JP, Siemes C, Aarnoudse AJ, Verhamme KM, Stricker BH et al (2008) Adverse drug reaction-related hospitalisations: a population-based cohort study. Pharmacoepidemiol Drug Saf 17(4):365–371
Wasserfallen J, Livio F, Buclin T, Tillet L, Yersin B, Biollaz J (2001) Rate, type, and cost of adverse drug reactions in emergency department admissions. Eur J Intern Med 12(5):442–447
Bouvy JC, De Bruin ML, Koopmanschap MA (2015) Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf 38(5):437–453
Beijer HJM, De Blaey CJ (2002) Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci 24(2):46–54
Kongkaew C, Noyce PR, Ashcroft DM (2008) Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother 42(7):1017–1025
Winterstein AG, Sauer BC, Hepler CD, Poole C (2002) Preventable drug-related hospital admissions. Ann Pharmacother 36(7–8):1238–1248
Patel TK, Patel PB (2016) Incidence of adverse drug reactions in Indian hospitals: a systematic review of prospective studies. Curr Drug Saf 11(2):128–136
Davies EA, O’Mahony MS (2015) Adverse drug reactions in special populations – the elderly. Br J Clin Pharmacol 80(4):796–807
Oscanoa TJ, Lizaraso F, Carvajal A (2017) Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol 73(6):759–770
By the American Geriatrics Society (2015) Beers criteria update expert panel (2015) American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 63(11):2227–2246
Jolivot PA, Hindlet P, Pichereau C, Fernandez C, Maury E, Guidet B et al (2014) A systematic review of adult admissions to ICUs related to adverse drug events. Crit Care 18(6):643
Stang AS, Wingert AS, Hartling L, Plint AC (2013) Adverse events related to emergency department care: a systematic review. PLoS One 8(9):e74214
Lisha J, Annalakshmi V, Maria J, Padmini D (2017) Adverse drug reactions in critical care setting: a systematic review. Curr Drug Saf 12(3):147–161
Hakkarainen KM, Hedna K, Petzold M, Hägg S (2012) Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions – a meta-analysis. PLoS One 7(3):e33236
Molokhia M, Tanna S, Bell D (2009) Improving reporting of adverse drug reactions: systematic review. Clin Epidemiol 1:75–92
Lessing C, Schmitz A, Albers B, Schrappe M (2010) Impact of sample size on variation of adverse events and preventable adverse events: systematic review on epidemiology and contributing factors. Qual Saf Health Care 19(6):e24
Snipelisky D, Kusumoto F (2013) Current strategies to minimize the bleeding risk of warfarin. J Blood Med 4:89–99
Lip GY, Frison L, Halperin JL, Lane DA (2011) Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly) score. J Am Coll Cardiol 57(2):173–180
Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY (2012) Performance of the HEMORR(2)HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol 60(9):861–867
Almutairi AR, Zhou L, Gellad WF, Lee JK, Slack MK, Martin JR et al (2017) Effectiveness and safety of non-vitamin K antagonist oral anticoagulants for atrial fibrillation and venous thromboembolism: a systematic review and meta-analyses. Clin Ther 39(7):1456–1478.e36
Caldeira D, Rodrigues FB, Barra M, Santos AT, de Abreu D, Gonçalves N et al (2015) Non-vitamin K antagonist oral anticoagulants and major bleeding-related fatality in patients with atrial fibrillation and venous thromboembolism: a systematic review and meta-analysis. Heart 101(15):1204–1211
van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV (2014) Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost 12(3):320–328
Elwood PC, Morgan G, Galante J, Chia JW, Dolwani S, Graziano JM et al (2016) Systematic review and meta-analysis of randomised trials to ascertain fatal gastrointestinal bleeding events attributable to preventive low-dose aspirin: no evidence of increased risk. PLoS One 11(11):e0166166
García Rodríguez LA, Martín-Pérez M, Hennekens CH, Rothwell PM, Lanas A (2016) Bleeding risk with long-term low-dose aspirin: a systematic review of observational studies. PLoS One 11(8):e0160046
Valkhoff VE, Sturkenboom MC, Kuipers EJ (2012) Risk factors for gastrointestinal bleeding associated with low-dose aspirin. Best Pract Res Clin Gastroenterol 26(2):125–140
Lanas A, Scheiman J (2007) Low-dose aspirin and upper gastrointestinal damage: epidemiology, prevention and treatment. Curr Med Res Opin 23:163–173
Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H et al (2011) ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 32(23):2999–3054
Vamos M, Erath JW, Hohnloser SH (2015) Digoxin-associated mortality: a systematic review and meta-analysis of the literature. Eur Heart J 36(28):1831–1838
Ouyang AJ, Lv YN, Zhong HL, Wen JH, Wei XH, Peng HW et al (2015) Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am J Cardiol 115(7):901–906
Ziff OJ, Lane DA, Samra M, Griffith M, Kirchhof P, Lip GY et al (2015) Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ 351:h4451
Hanif K, Bid HK, Konwar R (2010) Reinventing the ACE inhibitors: some old and new implications of ACE inhibition. Hypertens Res 33(1):11–21
Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou W et al (2016) Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis 67(5):728–741
Schmidt M, Mansfield KE, Bhaskaran K, Nitsch D, Sørensen HT, Smeeth L et al (2017) Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 356:j791
Lesogor A, Cohn JN, Latini R, Tognoni G, Krum H, Massie B et al (2013) Interaction between baseline and early worsening of renal function and efficacy of renin-angiotensin-aldosterone system blockade in patients with heart failure: insights from the Val-HeFT study. Eur J Heart Fail 15(11):1236–1244
Valle R, Aspromonte N, Milani L, Peacock FW, Maisel AS, Santini M et al (2011) Optimizing fluid management in patients with acute decompensated heart failure (ADHF): the emerging role of combined measurement of body hydration status and brain natriuretic peptide (BNP) levels. Heart Fail Rev 16(6):519–529
Kelly J, Chamber J (2000) Inappropriate use of loop diuretics in elderly patients. Age Ageing 29(6):489–493
Agal S, Baijal R, Pramanik S, Patel N, Gupte P, Kamani P et al (2005) Monitoring and management of antituberculosis drug induced hepatotoxicity. J Gastroenterol Hepatol 20(11):1745–1752
Abbara A, Chitty S, Roe JK, Ghani R, Collin SM, Ritchie A et al (2017) Drug-induced liver injury from antituberculous treatment: a retrospective study from a large TB centre in the UK. BMC Infect Dis 17(1):231
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors each declare that they do not have a conflict of interest.
Sponsor(s) of the research
This study has no sponsors.
Electronic supplementary material
Supplementary Figure 1
Risk of bias summary (PNG 40 kb)
Supplementary Figure 2
Funnel plot (JPEG 45 kb)
Supplementary Figure 3
Meta-analytic summary of prevalence of fatal ADRAd according to age (JPEG 960 kb)
Supplementary Figure 4
Meta-analytic summary of subgroup analysis of prevalence of fatal ADRAd according to study wards (JPEG 1154 kb)
ESM 1
(PDF 473 kb)
ESM 2
(PDF 208 kb)
ESM 3
(PDF 190 kb)
ESM 4
(PDF 783 kb)
Rights and permissions
About this article
Cite this article
Patel, T.K., Patel, P.B. Mortality among patients due to adverse drug reactions that lead to hospitalization: a meta-analysis. Eur J Clin Pharmacol 74, 819–832 (2018). https://doi.org/10.1007/s00228-018-2441-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2441-5