Abstract
Purpose
To explore, in a microdose (phase-0) study, the pharmacokinetics, bioavailability and concentrations in key compartments of the lung, of AR-709, a novel diaminopyrimidine antibiotic for the treatment of respiratory infection.
Methods
Four healthy men each received two single, 100 μg microdoses of 14C-AR-709, 7 days apart: the first was administered intravenously (IV), the second orally. Plasma pharmacokinetics of 14C and unchanged AR-709 were obtained by high-performance liquid chromatography and accelerator mass spectrometry (AMS). Next, 15 healthy men received a single, 100 μg microdose of 14C-AR-709 IV. Plasma, bronchoalveolar lavage fluid, alveolar macrophages and bronchial mucosal biopsy samples were analysed by AMS.
Results
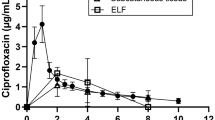
After IV administration, clearance of AR-709 was 496 mL/min, volume of distribution was 1,700 L and the absolute oral bioavailability was 2.5 %. Excretion in urine was negligible. At 8–12 h after IV dosing, 14C concentrations in lung samples were 15- (bronchial mucosa) to 200- (alveolar macrophages) fold higher than in plasma. In alveolar macrophages, 14C was still mostly associated with AR-709 at 12 h after dosing.
Conclusions
The results of this microdose study indicate that AR-709 attains concentrations appreciably higher within the lung than in plasma. Its low oral bioavailability however, precludes oral administration. Although IV administration would appear to be an effective route of administration, this would limit the use of AR-709 to a clinical setting and would therefore be economically unsustainable. If further clinical development were to be undertaken, therefore, an alternative route of administration would be necessary.




Similar content being viewed by others
References
Musher DM (1992) Infections caused by streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin Infect Dis 14(4):801–807
Mukhija S, Bandera M, Parisi S, Rigo S, Lieb S, Lociuro S, Gillessen D, Islam K (2006) AR709—an investigational diaminopyrimidine: inhibition, binding and mode of action. In: 46th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy. San Fransisco, Abstr F1-1955
Jansen WT, Verel A, Verhoef J, Milatovic D (2008) In vitro activity of AR-709 against Streptococcus pneumoniae. Antimicrob Agents Chemother 52(3):1182–1183
Ressner RA, Moore MR, Jorgensen JH (2008) Activity of the diaminopyrimidine AR-709 against recently collected multidrug-resistant isolates of invasive Streptococcus pneumoniae from North America. Antimicrob Agents Chemother 52(3):1147–1149
Smith K, Ednie LM, Appelbaum PC, Hawser S, Lociuro S (2008) Antistreptococcal activity of AR-709 compared to that of other agents. Antimicrob Agents Chemother 52(6):2279–2282
McKenney D, Murphy T, Little S, Gordon L, Islam K, Lociuro S (2007) Efficacy of AR-709 in pneumococcal murine pneumonia. In: 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy. Chicago
Lappin G (2010) Microdosing: current and the future. Bioanalysis 2(3):509–517
Lappin G, Garner RC (2003) Big physics, small doses: the use of AMS and PET in human microdosing of development drugs. Nat Rev Drug Discov 2(3):233–240
Li RC, Zhu M, Schentag JJ (1999) Achieving an optimal outcome in the treatment of infections. The role of clinical pharmacokinetics and pharmacodynamics of antimicrobials. Clin Pharmacokinet 37(1):1–16
Committee for Medicinal Products for Human Use (CPMP)/International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) (2009) ICH Topic M3 Note for Guidance on non-clinical safety pharmacology studies for human pharmaceuticals. CPMP/ICH/286/95. CPMP/ICH, London/Geneva
Lappin G, Wagner C, Langer O, Merbel VD (2009) New ultrasensitive detection technologies and techniques for use in microdosing studies. Bioanalysis 1(2):357–366
The BAL Cooperative Group Steering Committee (1990) Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. Am Rev Respir Dis 141:S166–S202
Turteltaub KW, Felton JS, Gledhill BL, Vogel JS, Southon JR, Caffee MW, Finkel RC, Nelson DE, Proctor ID, Davis JC (1990) Accelerator mass spectrometry in biomedical dosimetry: relationship between low-level exposure and covalent binding of heterocyclic amine carcinogens to DNA. Proc Natl Acad Sci USA 87(14):5288–5292
Gunnarsson M, Leide-Svegborn S, Stenstrom K, Skog G, Nilsson LE, Hellborg R, Mattsson S (2002) No radiation protection reasons for restrictions on 14C urea breath tests in children. Br J Radiol 75(900):982–986
Committee for Medicinal Products for Human Use (2004) Position paper on non-clinical safety studies to support clinical trials with a single microdose. Position paper CPMP/SWP/2599. European Medicines Agency, London
Albert A, Brown DJ (1954) Purine studies part I. Stability to acid and alkali. Solubility, ionization, comparison with pteridines. J Chem Soc 2060–2071
Bernatek E, Hvatum M (1960) On the stability of ozonides. Acta Chem Scand 14:836–840
Wenkert E, Alonso ME, Gottlib HE, Sanchez EL, Pellicciari R, Cogolli P (1977) Reactions of ethyl diazoacetate with thianaphthene, indoles and benzofuran. J Org Chem 42(24):3945–3949
Lappin G, Simpson M, Shishikura Y, Garner C (2008) High-performance liquid chromatography accelerator mass spectrometry: correcting for losses during analysis by internal standardization. Anal Biochem 378:93–95
Klech H, Pohl W (1989) Report of the European Society of Pneumology Task Group on BAL. Technical recommendations and guidelines for bronchoalveolar lavage (BAL). Eur Respir J 2(561–585)
Rennard SI, Basset G, Lecossier D, O’Donnell KM, Pinkston P, Martin PG, Crystal RG (1986) Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 60:532–538
Lappin G, Seymour M, Young G, Higton D, Hill HM (2011) An AMS method to determine analyte recovery from pharmacokinetic studies with concomitant extravascular and intravenous administration. Bioanalysis 3(4):407–410
Lappin G, Garner RC (2003) Ultra-sensitive detection of radiolabelled drugs and their metabolites using accelerator mass spectrometry. In: Wilson I (ed) Handbook of analytical separations, vol 4. Bioanalytical separations. Elsevier, Amsterdam, pp 331–349
Lappin G, Garner RC (2004) Current perspectives of 14C-isotope measurement in biomedical accelerator mass spectrometry. Anal Biochem 378(2):356–364
Salehpour M, Possnert G, Bryhni H (2008) Subattomole sensitivity in biological accelerator mass spectrometry. Anal Chem 80(10):3515–3521
Rowland M (2012) Microdosing: a critical assessment of human data. J Pharm Sci 101(11):4067–4074
Rowland M, Benet LZ (2011) Clinical trials and translational medicine commentaries. J Pharm Sci 100:4047–4049
Lappin G, Noveck R, Burt T (2013) Microdosing and drug development: past, present and future. Expert Opin Drug Metab Toxicol 9 (7):in press
Benet LZ, Broccatelli F, Oprea TI (2011) BDDCS applied to over 900 drugs. AAPS J 13(4):519–547
Lappin G, Shishikura Y, Jochemsen R, Weaver RJ, Gesson C, Brian Houston J, Oosterhuis B, Bjerrum OJ, Grynkiewicz G, Alder J, Rowland M, Garner C (2011) Comparative pharmacokinetics between a microdose and therapeutic dose for clarithromycin, sumatriptan, propafenone, paracetamol (acetaminophen), and phenobarbital in human volunteers. Eur J Pharm Sci 43:141–150
Conte JE Jr, Golden JA, Kipps J, Zurlinden E (2004) Steady-state plasma and intrapulmonary pharmacokinetics and pharmacodynamics of cethromycin. Antimicrob Agents Chemother 48(9):3508–3515
Periti P, Mazzei T, Mini E, Novelli A (1989) Clinical pharmacokinetic properties of the macrolide antibiotics. Effects of age and various pathophysiological states (Part II). Clin Pharmacokinet 16(5):261–282
Hii JT, Duff HJ, Burgess ED (1991) Clinical pharmacokinetics of propafenone. Clin Pharmacokinet 21(1):1–10
Zanni GR, Wick JY (2006) Microdosing: the new pharmacokinetic paradigm? Consult Pharm 21(10):756–776
Conflict of interest statement
The authors had support from Arpida Ltd. (Reinach, Switzerland) for the work reported here. There were no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years. No other relationships or activities have influenced the submitted work
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lappin, G., Boyce, M.J., Matzow, T. et al. A microdose study of 14C-AR-709 in healthy men: pharmacokinetics, absolute bioavailability and concentrations in key compartments of the lung. Eur J Clin Pharmacol 69, 1673–1682 (2013). https://doi.org/10.1007/s00228-013-1528-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1528-2