Abstract
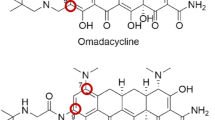
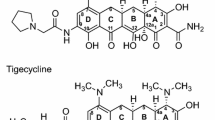
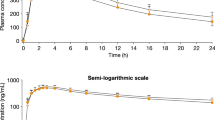
Omadacycline is a novel aminomethylcycline antibiotic (antibacterial). Omadacycline has had chemical structure modifications at the C9 and C7 positions of the core tetracycline rings that allow stability in the efflux pump and ribosomal protection protein mechanisms of tetracycline resistance. The systemic exposure (i.e., maximum plasma concentrations [Cmax] and area under the plasma concentration–time curve [AUC]) after intravenous (IV) administration were linear and predictable over the dose range of 25 and 600 mg in healthy subjects. The oral bioavailability of omadacycline was 34.5% under fasted conditions (no food intake 6 h before and 4 h after dosing). Both AUC and Cmax values significantly decreased (41–61%) when a high-fat meal, with and without dairy, were administered 2 h before oral dosing of omadacycline. Similar to other tetracyclines, it is advisable to avoid concurrent administration of divalent- or trivalent cation-containing products (e.g., antacids and iron-containing preparations) for at least 4 h after oral administration of omadacycline. Omadacycline has a large volume of distribution (190 L) and low plasma protein binding (21.3%) that was concentration independent. Systemic exposure of omadacycline in epithelial lining fluid (ELF) and alveolar macrophages was greater than in plasma in healthy adult subjects. Omadacycline is excreted unchanged in the feces (81.1%) and urine (14.4%), and has a low potential for drug–drug interactions since it was not a substrate, inhibitor, or inducer of major cytochrome-metabolizing enzymes or organic anion transporters (OATs). No clinically significant differences in the pharmacokinetics of omadacycline have been observed for age, sex, and renal or hepatic impairment. Pharmacokinetic–pharmacodynamic studies have confirmed that the AUC from time zero to 24 h (AUC24)/minimum inhibitory concentration (MIC) ratio was the best index for correlating unbound plasma and total-drug ELF concentrations with the efficacy of omadacycline. A population pharmacokinetic model was developed with data from healthy subjects and infected patients and used to establish interpretive criteria for in vitro susceptibility testing and dosing regimens of omadacycline for treating acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia.



Similar content being viewed by others
References
Burgos RM, Rodvold KA. Omadacycline: a novel aminomethylcycline. Infect Drug Resist. 2019;12:1895–915.
Zhanel GG, Cheung D, Adam H, Zelenitsky S, Golden A, Schweizer F, et al. Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs. 2016;76:567–88.
Zhanel GG, Hornenuik K, Nichol K, Noreddin A, Vercaigne L, Embil J, et al. The glycylcyclines: a comparative review with the tetracyclines. Drugs. 2004;64:63–88.
Tanaka SK, Steenbergen J, Villano S. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg Med Chem. 2016;24:6409–19.
Villano S, Steenbergen J, Loh E. Omadacycline: development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections. Future Microbiol. 2016;11:1421–34.
Karlowsky JA, Steenbergen J, Zhanel GG. Microbiology and preclinical review of omadacycline. Clin Infect Dis. 2019;69(Suppl 1):S6–15.
Honeyman L, Ismail M, Nelson ML, Bhatia B, Bowser TE, Chen J, et al. Structure-activity relationship of the aminomethylcyclines and the discovery of the omadacycline. Antimicrob Agents Chemother. 2015;59:7044–53.
Draper MP, Weir S, Macone A, Donatelli J, Trieber CA, Tanaka SK, et al. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob Agents Chemother. 2014;58:1279–83.
Markham A, Keam SJ. Omadacycline: first global approval. Drugs. 2018;78:1931–7.
Nuzyra (omadacycline) prescribing information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209816_209817lbl.pdf. Accessed 1 Jun 2019.
Kunin CM, Dornbush AC, Finland M. Distribution and excretion of four tetracycline analogues in normal young men. J Clin Invest. 1959;38:1950–63.
Steigbigel NH, Reed CW, Finland M. Absorption and excretion of five tetracycline analogues in normal young men. Am J Med Sci. 1968;255:296–312.
Wood MJ, Farrell W, Kattan S, Williams JD. Activity of minocycline and tetracycline against respiratory pathogens related to blood levels. J Antimicrob Chemother. 1975;1:323–31.
Sjölin-Forsberg G, Hermansson J. Comparative bioavailability of tetracycline and lymecycline. Br J Clin Pharmacol. 1984;18:529–33.
Welling PG, Koch PA, Lau CC, Craig WA. Bioavailability of tetracycline and doxycycline in fasted and nonfasted subjects. Antimicrob Agents Chemother. 1977;11:462–9.
Ylitalo P, Hinkka H, Neuvonen PJ. Effect of exercise on the serum level and urinary excretion of tetracycline, doxycycline and sulphamethizole. Eur J Clin Pharmacol. 1977;12:367–73.
Dimmling T, Vanderbeke O. Pharmacokinetics after oral and IV administration of tetracycline compounds. Medizinische Klinik. 1975;70:279–85.
Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet. 1988;15:355–66.
Fourtillan JB, Lefebrre MA, Saux MC, et al. Etude pharmacocinetique de la doxycycline chez l’home par chromatographie liquide haute performance. La Nourelle Presse Medicale. 1980;9:77–81.
Heaney D, Eknoyan G. Minocycline and doxycycline kinetics in chronic renal failure. Clin Pharmacol Ther. 1978;24:233–9.
Nguyen VX, Nix DE, Gillikin S, Schentag JJ. Effect of oral antacid administration on the pharmacokinetics of intravenous doxycycline. Antimicrob Agents Chemother. 1989;33:434–6.
Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother. 2006;58:256–65.
Bistue C, Perez P, Becquart D, Vinçon G, Albin H. The effect of dimethicone on the bioavailability of doxycycline. Therapie. 1987;42:13–6.
Campistron G, Coulais Y, Caillard C, Mosser J, Pontagnier H, Houin G. Pharmacokinetics and bioavailability of doxycycline in humans. Arzneimittelforschung. 1986;36:1705–7.
Houin G, Brunner F, Nebout T, Cherfaoui M, Lagrue G, Tillement JP. The effects of chronic renal insufficiency on the pharmacokinetics of doxycycline in man. Br J Clin Pharmacol. 1983;16:245–52.
Wójcicki J, Kalinowski W, Gawrońska-Szklarz B. Comparative pharmacokinetics of doxycycline and oxytetracycline in patients with hyperlipidemia. Arzneimittelforschung. 1985;35:991–3.
Cornely OA, Arenz D, Barraud O, Bayliss M, Dimitriou V, Lovering AM, et al. Phase 1 study to evaluate the safety and pharmacokinetics of single and multiple ascending doses of intravenous minocycline in healthy adult subjects [poster no. P1387]. 2018. In: IDWeek Annual Meeting; San Francisco.
Macdonald H, Kelly RG, Allen ES, Noble JF, Kanegis LA. Pharmacokinetic studies on minocycline in man. Clin Pharmacol Ther. 1973;14:852–61.
Cartwright AC, Hatfield HL, Yeadon A, London E. A comparison of the bioavailability of minocycline capsules and film-coated tablets. J Antimicrob Chemother. 1975;1:317–22.
Conte JE Jr, Golden JA, Kelly MG, Zurlinden E. Steady-state serum and intrapulmonary pharmacokinetics and pharmacodynamics of tigecycline. Int J Antimicrob Agents. 2005;25:523–9.
Tygacil. US prescribing information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211158s000lbl.pdf. Accessed 2 Jun 2019.
Newman JV, Zhou J, Izmailyan S, Tsai L. Randomized, double-blind, placebo-controlled studies of the safety and pharmacokinetics of single and multiple ascending doses of eravacycline. Antimicrob Agents Chemother. 2018;62:e01174-18.
Xerava. US prescribing information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211109lbl.pdf. Accessed 2 Jun 2019.
Berg JK, Tzanis E, Garrity-Ryan L, Bai S, Chitra S, Manley A, et al. Pharmacokinetics and safety of omadacycline in subjects with impaired renal function. Antimicrob Agents Chemother. 2018;62(2):e02057-17.
Flarakos J, Du Y, Gu H, Wang L, Einolf HJ, Chun DY, et al. Clinical disposition, metabolism and in vitro drug–drug interaction properties of omadacycline. Xenobiotica. 2017;47:682–96.
Bundrant LA, Tzanis E, Garrity-Ryan L, Bai S, Chitra S, Manley A, et al. Safety and pharmacokinetics of the aminomethylcycline antibiotic omadacycline administered to healthy subjects in oral multiple-dose regimens. Antimicrob Agents Chemother. 2018;62:e01487-17.
Tzanis E, Manley A, Villano S, Tanaka SK, Bai S, Loh E. Effect of food on the bioavailability of omadacycline in healthy participants. J Clin Pharmacol. 2017;57:321–7.
Villano SJ, Tzanis E, Tanaka SK. In vitro protein binding with omadacycline, a first in class aminomethylcycline antibiotic [poster no. 5180]. 2016. American Society of Microbiology Microbe Annual Meeting; Boston.
Zhou J, Tran B, Tam VH. The complexity of minocycline serum protein binding. J Antimicrob Chemother. 2017;72:1632–4.
Macone AB, Caruso BK, Leahy RG, Donatell J, Weir S, Draper MP, et al. In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob Agents Chemother. 2014;58:1127–35.
Pfaller MA, Huband MD, Shortridge D, Flamm RK. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe as part of the 2016 SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother. 2018;62:e02337-17.
Huband MD, Pfaller MA, Shortridge D, Flamm RK. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe: results from the SENTRY Antimicrobial Surveillance Programme 2017. J Glob Antimicrob Resist. 2019;19:56–63. https://doi.org/10.1016/j.jgar.2019.02.017.
Kohlhoff SA, Huerta N, Hammerschlag MR. In vitro activity of omadacycline against Chlamydia pneumoniae. Antimicrob Agents Chemother. 2019;63:e01907–18.
Waites KB, Crabb DM, Liu Y, Duffy LB. In vitro activities of omadacycline (PTK 0796) and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob Agents Chemother. 2016;60:7502–4.
O’Riordan, Green S, Overcash JS, Puljiz I, Metallidis S, Gardovskis J, et al. Omadacycline for acute bacterial skin and skin-structure infections. N Engl J Med. 2019;380:528–38.
O’Riordan W, Cardenas C, Shin E, Sirbu A, Garrity-Ryan L, Das AF, et al. Once-daily oral omadacycline versus twice-daily oral linezolid for acute bacterial skin and skin structure infections (OASIS-2): a phase 3, double-blind, multicenter, randomized, controlled, non-inferiority trial. Lancet Infect Dis. 2019;19(10):1080–90. https://doi.org/10.1015/S147-3099(19)30275-0.
Stets R, Popescu M, Gonong JR, Mitha I, Nseir W, Madej A, et al. Omadacycline for community-acquired bacterial pneumonia. N Engl J Med. 2019;380:517–27.
Rameriz JA, Tzanis E, Curran M, Noble R, Chitra S, Manley A, et al. Early clinical response in community-acquired bacterial pneumonia: from clinical endpoint to clinical practice. Clin Infect Dis. 2019;69(Suppl 1):S33–9.
Stapert L, Wolfe C, Shinabarger D, Marra A, Pillar C. In vitro activity of omadacycline and comparators against anaerobic bacteria. Antimicrob Agents Chemother. 2018;62:e00047-18.
Pfaller MA, Rhomberg PR, Huband MD, Flamm RK. Activity of omadacycline tested against Enterobacteriaceae causing urinary tract infections from a global surveillance program (2014). Diagn Microbiol Infect Dis. 2018;91:179–83.
Goldstein EJC, Citron DM, Tyrrell KL, Leoncio E, Merriam CV. Comparative in vitro activity of omadacycline against dog and cat bite would isolates. Antimicrob Agents Chemother. 2018;62:e02551-17.
Bax HI, de Vogel CP, Mouton JW, de Steenwinkel JEM. Omadacycline as a promising new agent for the treatment of infections with Mycobacterium abscessus. J Antimicrob Chemother. 2019;74(10):2930–3. https://doi.org/10.1093/jac/dkz267.
Kaushik A, Ammerman NC, Martins O, Parrish NM, Nuemberer EL. In vitro activity of new tetracycline analogs omadacycline and eravacycline against drug-resistant clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother. 2019;63:e00470-19.
Shoen C, Benaroch D, Sklaney M, Cynamon M. In vitro activities of omadacycline against rapidly growing mycobacteria. Antimicrob Agents Chemother. 2019;63:e02522-18.
Steenbergen J, Tanaka SK, Miller LL, Halasohoris SA, Hershfield JR. In vitro and in vivo activity of omadacycline against two biothreat pathogens Bacillus anthracis and Yersinia pestis. Antimicrob Agents Chemother. 2017;61:e02434-16.
Carvalhases CG, Huband MD, Reinhart HH, Flamm RK, Sader HS. Antimicrobial activity of omadacycline tested against clinical bacterial isolates from hospitals in mainland China, Hong Kong, and Taiwan: results from the SENTRY Antimicrobial Surveillance Program (2013 to 2016). Antimicrob Agents Chemother. 2019;63:e02262-18.
Pfaller MA, Rhomberg PR, Huband MD, Flamm RK. Activity of omadacycline tested against Streptococcus pneumoniae from a global surveillance program (2014). Diagn Microbiol Infect Dis. 2018;90:143–7.
Pfaller MA, Huband MD, Rhomberg PR, Flamm RK. Surveillance of omadacycline activity against clinical isolates from a global collection (North America, Europe, Latin America, Asia-Western Pacific) 2010–2011. Antimicrob Agents Chemother. 2017;61:e00018-17.
Pfaller MA, Rhomberg PR, Huband MD, Flamm RK. Activities of omadacycline and comparator agents against Staphylococcus aureus isolates from a surveillance program conducted in North America and Europe. Antimicrob Agents Chemother. 2017;61:e02411–6.
Ting L, Sun H, Kovacs SJ, Klausner K, Tanaka K. Pharmacokinetics of intravenous and oral PTK796, a new aminomethylcycline antibiotics [abstract no. 1281]. 2010. 50th Annual Interscience Conference of Antimicrobial Agents and Chemotherapy; Boston.
Sun H, Ting L, Machineni S, Praestgaard J, Kuemmell A, Stein DS, et al. Randomized, open-label study of the pharmacokinetics and safety of oral and intravenous administration of omadacycline to healthy subjects. Antimicrob Agents Chemother. 2016;60:7431–5.
Sun H, Ting L, Flarakos J, Dole K, Praestgaard J, Kovas SJ, et al. Pharmacokinetics of [14C]-labeled omadacycline (PTK0796) in healthy male subjects [abstract 1281]. 2012. 52nd Annual Interscience Conference of Antimicrobial Agents and Chemotherapy; San Francisco, CA.
Overcash JS, Bhiwandi P, Garrity-Ryan L, Steenbergen J, Bai S, Chitra S, et al. Pharmacokinetics, safety, and clinical outcomes of omadacycline in women with cystitis: results from a phase 1b study. Antimicrob Agents Chemother. 2019;63:e02083-18.
Tanaka SK, Villano S, Tzanis E. Single and multiple dose pharmacokinetics and tolerability of intravenous omadacycline in healthy volunteers [poster no. P1319]. 2016. European Conference on Clinical Microbiology and Infectious Diseases Annual Meeting; Amsterdam.
Gotfried MH, Horn K, Garrity-Ryan L, Villano S, Tzanis E, Chitra S, et al. Comparison of omadacycline and tigecycline pharmacokinetics in the plasma, epithelial lining fluid, and alveolar cells of healthy adult subjects. Antimicrob Agents Chemother. 2017;61:e01135-17.
Lin W, Flarakos J, Du Y, Hu W, He H, Mangold J, et al. Pharmacokinetics, distribution, metabolism, and excretion of omadacycline following a single intravenous or oral dose of 14C-omadacycline in rats. Antimicrob Agents Chemother. 2017;61:e01784-16.
Lakota EA, Van Wart SA, Tzanis E, Bhavnani SM, Ambrose PG, Rubino CM. Population pharmacokinetic analyses of omadacycline using phase 1 and 3 data [poster no. 628]. American Society of Microbiology Microbe Annual Meeting; 7–11 Jun 2018; Atlanta.
Smith C, Woods CG, Woods MJ. Absorption of minocycline. J Antimicrob Chemother. 1984;13:93.
Sulsa GM. Miscellaneous antibiotics. In: Pai MP, Kiser JJ, Gubbins PO, Rodvold KA, editors. Drug interactions in infectious diseases: antimicrobial drug interactions. 4th ed. Cham: Humana Press; 2018. p. 167–220.
Van Wart SA, Manley A, Bhavnani SM, Tanaka K, Loh E, Rubino CM, et al. Population pharmacokinetics of omadacycline following intravenous or oral administration to phase 1 subjects [poster no. P1320]. 2016. 26th European Congress of Clinical Microbiology and Infectious Diseases; Amsterdam.
Van Wart SA, Manley A, Bhavnani SM, Tanaka K, Loh E, Tzanis E, et al. Population pharmacokinetics of omadacycline following intravenous and oral administration and evaluation of phase 3 sparse PK sampling strategies [poster no. Monday-509]. 2016. American Society of Microbiology (ASM) Microbe 2016; Boston.
Lakota EA, Rodvold KA, Bhavnani SM, Steenbergen JN, Tzanis E, Ambrose PG, et al. A pharmacometric comparison of omadacycline and tigecycline epithelial lining fluid penetration [poster no. 1850]. 2017. IDWeek; San Diego.
Lakota EA, Friedrich L, Steenbergen JN, McGovern PC, Tzanis E, Bhavnani SM, et al. Omadacycline pharmacokinetics: impact of comorbidities [poster no. P1943]. 2019. 29th European Congress of Clinical Microbiology and Infectious Diseases; Amsterdam.
Bhavnani SM, Hammel JP, Lakota EA, Liolios K, Rubino CM, Steenbergen JN, et al. Assessment of pharmacokinetics-pharmacodynamics to support omadacycline dosing regimens for the treatment of patients with acute bacteridal skin and skin structure infections [poster no. 1944]. 2019. 29th European Congress of Clinical Microbiology and Infectious Diseases; Amsterdam.
Paratek Pharmaceuticals. Omadacycline p-toluenesulfonate tablets and injection for the treatment of acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CAPD). Briefing Document for FDA Antimicrobial Drugs Advisory Committee. 2018. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM615849.pdf. Accessed 15 Jul 2019.
Bhavnani SM, Hammel JP, Lakota EA, Bader JC, Bulik CC, VanScoy BD, et al. Pharmacokinetic-pharmacodynamic target attainment analyses evaluating omadacycline dosing regimens for the treatment of patients with community-acquired bacterial pneumonia for Streptococcus penumoniae and Haemophilus influenzae [poster no. Saturday-625]. 2018. American Society of Microbiology Microbe; Atlanta.
Tanaka SK, Tzanis E, Villano S. Effect of age and gender on the pharmacokinetics of oral and IV omadacycline, a new class of aminomethylcyclines [poster no. P-1318]. 2016. 26th European Congress of Clinical Microbiology and Infectious Diseases; Amsterdam.
Ting L, Kovacs SJ, Praestgaard J, Maietta R, Stein DS, Sunkara G, et al. Pharmacokinetics of omadacycline (PTK0796) in subjects with hepatic impairment [abstract no. 1282]. 2012. 52nd Annual Interscience Conference of Antimicrobial Agents and Chemotherapy; San Francisco.
Craig WA, Andes D, Odinecs A. In vivo pharmacodynamics of MK-2764/PTK 0796 against Gram-positive and Gram-negative bacteria in the thighs of neutropenic and normal mice [abstract no. 1875]. 2006. 46th Annual Interscience Conference of Antimicrobial Agents and Chemotherapy; San Francisco.
Lepak AJ, Zhao M, Marchillo K, VanHecker J, Andes DR. In vivo pharmacodynamic evaluation of omadacycline (PTK 0796) against Streptococcus pneumoniae in the murine pneumonia model. Antimicrob Agents Chemother. 2017;61:e02368-16.
Lepak AJ, Zhao M, Marchillo K, VanHecker J, Andes DR. In vivo pharmacodynamics of omadacycline against Staphylococcus aureus in the neutropenic murine thigh infection model. Antimicrob Agents Chemother. 2019;63:e00624-19.
Tessier PR, Fan HW, Tanaka SK, Nicolau DP. Pharmacokinetic/pharmacodynamic profile of MK-2764/PTK 0796 against S. pneumoniae in a murine pneumonia model [abstract no. 1888]. In: 46th Annual Interscience Conference of Antimicrobial Agents and Chemotherapy; 2006.
VanScoy BD, Lakota EA, Conde H, Bhavnani SM, Steenbergen JN, Ambrose PG. Pharmacokinetic-pharmacodynamic characterization of omadacycline against Haemophilus influenzae using a one-compartment in vitro infection model [poster no. Saturday-624]. In: American Society of Microbiology Microbe; 2018.
Andes D, Craig WA. Pharmacokinetics and pharmacodynamics of tetracyclines. In: Nightingale CH, Ambrose PG, Drusano GL, Murakawa T, editors. Pharmacodynamics in theory and clinical practice. 2nd ed. New York: Informa Healthcare USA, Inc.; 2007. p. 267–77.
MacGowan AP. Tigecycline pharmacokinetic/pharmacodynamic update. J Antimicrob Chemother. 2008;62(Suppl 1):i11–6.
Hinshaw R, Stapert L, Shinabarger D, Pillar C. Post-antibiotic effect of omadacycline against target pathogens [poster no. Monday-512]. In: American Society of Microbiology Microbe; 2016.
VanScoy BD, Conde H, Tanaka K, Bhavnani SM, Steenbergen JN, Ambrose PG. Evaluation of the in vitro activity profile of omadacycline against Haemophilus influenzae [poster no. Monday-510]. In: American Society of Microbiology Microbe; 2016.
Macone AB, Donatelli JE, Draper MP, Tanaka SK. In vitro activity of omadacycline (PTK796) in broth, broth plus lung surfactant or human serum [poster no. P1141]. In: 21st European Congress of Clinical Microbiology and Infectious Diseases; 2011.
Opal S, File TM, van der Poll T, Tzanis E, Chitra S, McGovern PC. An integrated safety summary of omadacycline, a novel aminomethylcycline antibiotic. Clin Infect Dis. 2019;69(Suppl 1):S40–7.
Darpo B, Xue H, Tanaka SK, Tzanis E. A randomized, double-blind, placebo- and positive-controlled crossover study of the effects of omadacycline on QT/QTc intervals in healthy subjects. Antimicrob Agents Chemother. 2019;63:e00922-19. https://doi.org/10.1128/AAC.00922-19.
Tanaka Sk, Villano S. In vitro and in vivo assessments of cardiovascular effects with omadacycline. Antimicrob Agents Chemother. 2016;60:5247–53.
Omadacycline. Drugs and Lactation Database (LactMed). Bethesda: National Library of Medicine (US). 2006. https://www.ncbi.nlm.nih.gov/books/NBK535602/. Accessed 14 Nov 2019.
Cox EM. NDA 209816, NDA 209817, NDA approval. Department of Health and Human Services. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/209816Orig1s000,209817Orig1s000Ltr.pdf. Accessed 28 Oct 2019.
Funding
The authors received no financial compensation for the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KAR and MPP have served as consultants to Paratek Pharmaceuticals, Inc. No conflict of interests exist for RMB and XT.
Rights and permissions
About this article
Cite this article
Rodvold, K.A., Burgos, R.M., Tan, X. et al. Omadacycline: A Review of the Clinical Pharmacokinetics and Pharmacodynamics. Clin Pharmacokinet 59, 409–425 (2020). https://doi.org/10.1007/s40262-019-00843-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-019-00843-4